��Ŀ����
��22�֣�ij��ѧ��ѧ��ȤС������ȡ������ˮ����������ˮ������ʵ�顣����ʹ����ͼװ����ȡ�϶����ı�����ˮ����ش�
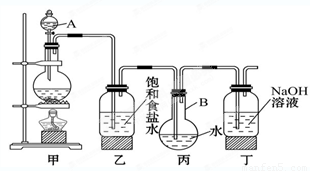
(1)д�����������ƣ�A_________�� B _________ ��
(2)д��������װ���з�����Ӧ�����ӷ���ʽ��
��_________________________________________________��
��_________________________________________________��
(3)��ͬѧ������¸Ľ����飺
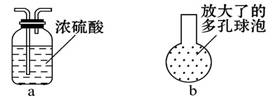
�����Һͱ�֮��������ͼ�е�aװ�ã�����Ϊ���ޱ�Ҫ_______ (��С����ޡ�)��
���ڱ��ij������¿ڴ�������ͼ�е�bװ�ã������������������Ч����ԭ����_______________________________ ____��
(4)���Ƶõ���ˮ�ֱ��������ʵ�飺�ٵ���̼������Һ�У����������ɣ�˵����ˮ�з�����Ӧ��������_____��
�ڵ���AgNO3��Һ�У�������Ӧ�����ӷ���ʽ____________________________________��
(5)�õιܽ��Ƶõı�����ˮ�������뺬��̪��NaOHϡ��Һ�С����ε����һ��ʱ��ɫͻȻ��ȥ���������������ԭ�����������(��Ҫ����˵��)��
��������__________________________________________��
��������__________________________________________��
��ʵ��֤����ɫ��ȥ��ԭ���Ǣٻ��ߢڵ�ʵ�鷽����__ ___
��
(6)Ϊ�������ˮ�д������Ũ�ȣ���ǿ��ˮ��Ư��������������ˮ�м�����Լ�_____(�����)��
A.CaCO3 B.Na2SO3 C.H2SO4 D.Ca(OH)2
��1����Һ©�� Բ����ƿ
��2��Cl2+H2O H++Cl-+HClO
Cl2��2OH��=Cl����ClO����H2O
H++Cl-+HClO
Cl2��2OH��=Cl����ClO����H2O
��3������ �ڿ�����Cl2��ˮ�ĽӴ���� (4) ��H+ ��Ag++Cl- ====AgCl��
(5) �����к����������� �����Ὣ��̪������ ����ɫ�����Һ���ٵμ�����������Һ������Һ��죬˵����ԭ��٣�����Һ�����˵����ԭ��� (6) A
����������1�����������Ľṹ�ص��֪��AB�ֱ��Ƿ�Һ©����Բ����ƿ��
��2�������ܺ�ˮ��Ӧ�����Ȼ���ʹ����ᣬ���Է���ʽΪCl2+H2O H++Cl-+HClO��Cl2��2OH��=Cl����ClO����H2O��
H++Cl-+HClO��Cl2��2OH��=Cl����ClO����H2O��
��3����aװ�õ�Ŀ���Ǹ������������������������Ʒ�Ӧ��û�б�Ҫ����ġ�
�ڸ���װ�õĽṹ��֪��ͨ��bװ�ÿ�����Cl2��ˮ�ĽӴ�������Ӷ��������Ч�ʡ�
��4�����ܺ�̼���Ʒ�Ӧ���������Ӧ����H����
��Cl���ܺ����������������Ȼ�������������ʽΪAg++Cl- ====AgCl����
��5�������ڼ�Һ���Ƿ�̪��Һ�Ժ�ɫ��������ʹ��̪��Һ��ɫ��ԭ���������к����������ƣ����Խ�������ġ�����Ϊ�������Һǿ�����ԣ��ܰѷ�̪��Һ��������ɫ��
����֤�ķ����Ǽ����μ�����������Һ��������ɫ�����Һ���ٵμ�����������Һ������Һ��죬˵����ԭ��٣�����Һ�����˵����ԭ��ڡ�
��6���������ܺ����������Լ��������Ʒ�Ӧ������ѡ��BD����ȷ��������ǿ�ᣬ������������ˮ�ķ�Ӧ��C����ȷ��̼����ܺ����ᷴӦ�����ʹ������Ӧ������ѡ��A��ȷ��


