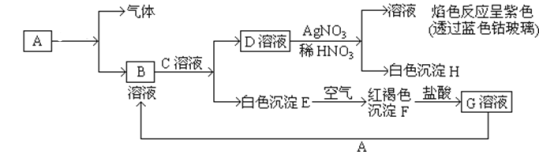
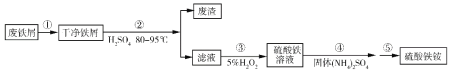
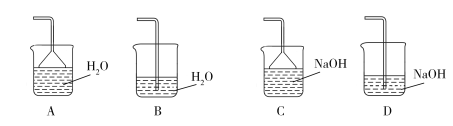
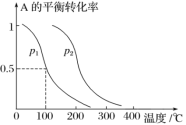
��Ŀ����
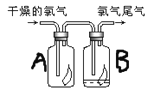
����Ŀ����1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿA��ʢ�г�ʪ��ɫ�����Ĺ��ƿB���ɹ۲쵽��������__�������������ԭ��___�����������ӷ���ʽ�ͼ����ֽ��ͣ�
��2��Ϊ��ֹ����β����Ⱦ������������ˮ�����Ե����ʣ�����__��Һ���ն����������ԭ����___���û�ѧ����ʽ��ʾ����
��3��������һԭ������ҵ�ϳ������۵�ʯ�������չ�ҵ����β���Ƶ�Ư�ۣ�Ư�۵���Ч�ɷ���__���ѧʽ��������¶���ڿ����е�Ư�ۻ���ʣ����ʺ��Ư�ۼ�ϡ����������������__������ĸ�������
A��O2 B��Cl2 C��CO2 D��HClO
���𰸡����ƿA�и������ɫ��������ɫ�����ƿB��ʪ�����ɫ������ɫ Cl2+H2O![]() H++Cl-+HClO�����������Ư���Զ�ʹ��ɫ������ɫ NaOH Cl2+2NaOH=NaCl+NaClO+H2O Ca(ClO)2 C
H++Cl-+HClO�����������Ư���Զ�ʹ��ɫ������ɫ NaOH Cl2+2NaOH=NaCl+NaClO+H2O Ca(ClO)2 C
��������
��1���������������ʹ�������ɫ������ɫ��ʪ�����ɫ�����к���ˮ��ˮ�ܹ���������Ӧ���ɴ����
��2����ˮ�����ԣ�����ѡ�ü��Ե�����������Һ�������β�����գ�
��3��Ư�۵���Ҫ�ɷ����Ȼ��ơ�������ƣ���Ч�ɷ��Ǵ�����ƣ�Ư��Ҫ�ܷⱣ�棬�������¶���ڿ����������ʡ�
��1������û��Ư���ԣ��������������ʹ���ƿA�и������ɫ������ɫ����������ƿB�г�ʪ����ɫ�����е�ˮ��Ӧ��Cl2+H2O![]() H++Cl-+HClO�����������Ư���Զ�ʹ��ɫ������ɫ��
H++Cl-+HClO�����������Ư���Զ�ʹ��ɫ������ɫ��
��2����ˮ�����ԣ�����������������Һ���ն������������Ӧ����ʽΪCl2+2NaOH=NaCl+NaClO+H2O��
��3��2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O��Ư�۵���Ч�ɷ���Ca(ClO)2������¶���ڿ����е�Ư�ۻ���ʣ�Ca(ClO)2+CO2+H2O=CaCO3��+2HClO��CaCO3��+2HCl= CaCl2+ CO2��+H2O���ʳ���¶���ڿ����б��ʺ��Ư�ۼ�ϡ����������������CO2����ѡC��