��Ŀ����
ijС�����ʵ�飬̽��NO2��ˮ���գ�����ﵽ����NO2������ȫ������ �ڼ���ƿ�в��������御�����٣�
��1��ʵ���ҳ���Cu��ŨHNO3��Ӧ����ȡ���仯ѧ����ʽΪ______
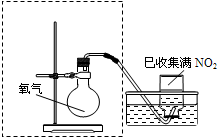
��2����ͼ���߿�����ijͬѧ��Ƶ�װ�ü�ͼ�����㲹�仭������ȱ�ٵ�һ�����������Լ���______��
��3��������Ƶ�ʵ������±���
��4����ԭ�������ڹ�ҵ�������ᣮΪ������Ⱦ����ŷţ��������һ����ʩ��______��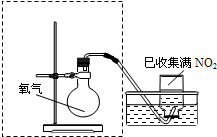
��1��ʵ���ҳ���Cu��ŨHNO3��Ӧ����ȡ���仯ѧ����ʽΪ______
��2����ͼ���߿�����ijͬѧ��Ƶ�װ�ü�ͼ�����㲹�仭������ȱ�ٵ�һ�����������Լ���______��
��3��������Ƶ�ʵ������±���
| ʵ�鲽�� | ʵ������ | ��ѧ����ʽ | |
| �� | ���ռ���NO2�ļ���ƿ���� ��ˮ���� |
||
| �� | ��������ɫ��ɺ���ɫ�� ���ֱ����ɫ��Һ��� ������ | ||
| �� | ��������������ͬ����� ƿ��ֻ�����������壬Һ �弸������ |

��1��Cu��Ũ���ᷴӦ��������ͭ������������ˮ���÷�ӦΪCu+4HNO3��Ũ��=Cu��NO3��2+2NO2��+2H2O���ʴ�Ϊ��Cu+4HNO3��Ũ��=Cu��NO3��2+2NO2��+2H2O��
��2���÷�ӦΪ������Һ��ķ�Ӧ����Ҫ��Һ©����Һ�壬��Һ©����ʢ��Ũ���ᣬ��ͼ
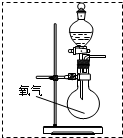
���ʴ�Ϊ��

��
��3��������������ˮ������3NO2+H2O=2HNO3+NO���۲쵽����ƿ��Һ������Լ����֮���������ɺ����ɫ�����ɫ�����в�����ͨ����������2NO+O2=2NO2���ظ������ڣ��ܷ�ӦΪ4NO2+O2+2H2O=4HNO3����Һ�弸����������ƿ��
�ʴ�Ϊ��
��4��Ϊ������Ⱦ����ŷţ���4NO2+O2+2H2O=4HNO3��֪��ˮ����NO2ʱ��ͨ���Թ����Ŀ�����ʹ�����������������ˮ��ȫ��Ӧ�������գ��ʴ�Ϊ��ˮ����NO2ʱ��ͨ���Թ����Ŀ�����ʹ�����������������ˮ��ȫ��Ӧ�������գ�
��2���÷�ӦΪ������Һ��ķ�Ӧ����Ҫ��Һ©����Һ�壬��Һ©����ʢ��Ũ���ᣬ��ͼ

���ʴ�Ϊ��

��
��3��������������ˮ������3NO2+H2O=2HNO3+NO���۲쵽����ƿ��Һ������Լ����֮���������ɺ����ɫ�����ɫ�����в�����ͨ����������2NO+O2=2NO2���ظ������ڣ��ܷ�ӦΪ4NO2+O2+2H2O=4HNO3����Һ�弸����������ƿ��
�ʴ�Ϊ��
| �� | ����ƿ��Һ������Լ���� ֮���������ɺ����ɫ�� ����ɫ |
3NO2+H2O=2HNO3+NO 2NO+O2=2NO2 | |
| �� | ��ˮ��ε�����ƿ����ʢ�� NO2�ļ���ƿͨ���������� ��ֹͣͨ�� |
||
| �� | ����ظ��ڢڲ����������� ��������ͨ������ֱ������ ͨ������岻��ɺ���ɫ |

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
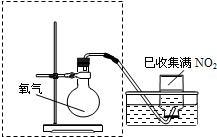 ijС�����ʵ�飬̽��NO2��ˮ���գ�����ﵽ����NO2������ȫ�����ա��ڼ���ƿ�в��������御�����٣�
ijС�����ʵ�飬̽��NO2��ˮ���գ�����ﵽ����NO2������ȫ�����ա��ڼ���ƿ�в��������御�����٣�
��1��ʵ���ҳ���Cu��ŨHNO3��Ӧ����ȡ���仯ѧ����ʽΪ______
��2����ͼ���߿�����ijͬѧ��Ƶ�װ�ü�ͼ�����㲹�仭������ȱ�ٵ�һ�����������Լ���______��
��3��������Ƶ�ʵ������±���
| ʵ�鲽�� | ʵ������ | ��ѧ����ʽ | |
| �� | ���ռ���NO2�ļ���ƿ���� ��ˮ���� | ||
| �� | ��������ɫ��ɺ���ɫ�� ���ֱ����ɫ��Һ��� ������ | ||
| �� | ��������������ͬ����� ƿ��ֻ�����������壬Һ �弸������ |
ijС�����ʵ�飬̽��NO2��ˮ���գ�����ﵽ����NO2������ȫ������ �ڼ���ƿ�в��������御�����٣�
��1��ʵ���ҳ���Cu��ŨHNO3��Ӧ����ȡ���仯ѧ����ʽΪ______
��2����ͼ���߿�����ijͬѧ��Ƶ�װ�ü�ͼ�����㲹�仭������ȱ�ٵ�һ�����������Լ���______��
��3��������Ƶ�ʵ������±���
��4����ԭ�������ڹ�ҵ�������ᣮΪ������Ⱦ����ŷţ��������һ����ʩ��______��
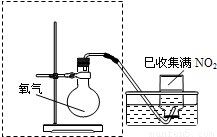
��1��ʵ���ҳ���Cu��ŨHNO3��Ӧ����ȡ���仯ѧ����ʽΪ______
��2����ͼ���߿�����ijͬѧ��Ƶ�װ�ü�ͼ�����㲹�仭������ȱ�ٵ�һ�����������Լ���______��
��3��������Ƶ�ʵ������±���
| ʵ�鲽�� | ʵ������ | ��ѧ����ʽ | |
| �� | ���ռ���NO2�ļ���ƿ���� ��ˮ���� | ||
| �� | ��������ɫ��ɺ���ɫ�� ���ֱ����ɫ��Һ��� ������ | ||
| �� | ��������������ͬ����� ƿ��ֻ�����������壬Һ �弸������ |

 ijС�����ʵ�飬̽��NO2��ˮ���գ�����ﵽ����NO2������ȫ������ �ڼ���ƿ�в��������御�����٣�
ijС�����ʵ�飬̽��NO2��ˮ���գ�����ﵽ����NO2������ȫ������ �ڼ���ƿ�в��������御�����٣�
 ij��ѧ��ȤС��Ϊ��̽���ڳ�����ij�ǽ����������γɵ�δ֪����ijɷ֣���С���Ա������ͨ�����ʯ��ˮ�����ֱ���ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������룮
ij��ѧ��ȤС��Ϊ��̽���ڳ�����ij�ǽ����������γɵ�δ֪����ijɷ֣���С���Ա������ͨ�����ʯ��ˮ�����ֱ���ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������룮