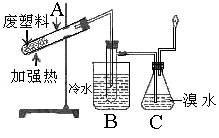
��Ŀ����

��05���Ϻ�������1�� ������Ԫ�����ڱ��л�������Ԫ����ǽ���Ԫ�صķֽ��ߡ�
��2�� ����NaH�Ĵ��ڣ���������ɷ���Ԫ�ط���VIIA�壬��ô�������������������۵ľ���ֵ��ȣ��ֿɷ���Ԫ�ط������ڱ��е� �塣
��3�� ���мס�������Ԫ�أ���Ԫ��ԭ�Ӻ���3p�Dz�����5�����ӣ���Ԫ�ص���ɫ��Ӧ�Ի�ɫ��
�� ��Ԫ�ط��Ž��ס�����Ԫ����д������Ԫ�����ڱ��ж�Ӧλ�á�
�� ��Ԫ������Ԫ����Ƚϣ��ǽ����Խ�ǿ���� �������ƣ���
д��������֤�ý��۵�һ����ѧ��Ӧ����ʽ ��
�𰸣���A�������8�֣���1��
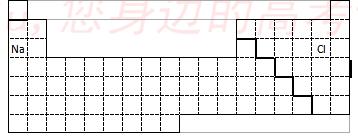
��2��IVA
��3�����ұ��� ���� H2S+Cl2��2HCl+S��

��ϰ��ϵ�д�
�����Ŀ