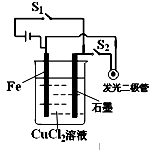
��Ŀ����
��6�֣�2011��6�£���������½������ʵҵ����˾5000��ֹ�ҵ���ϸ����Ƿ��㵹���µ���������Ⱦ����6�۸��ױ��������գ����°�����3�۸����ױ��������գ�����С����ҵ������ˮ�Ĵ�������֮һ�ǽ���+6�۸��ķ�ˮ��������ڣ�����������������������NaCl����е�⣺���������ɵ�Fe2+��Cr2O72��������Ӧ�����ɵ�Fe3+��Cr3+����������OH���������Fe(OH)3��Cr(OH)3������ȥ��
����֪��������Ksp Fe(OH)3=2.6��10��39��Ksp Cr(OH)3=6.0��10��31��
��1������������������16.8g���������ϱ���ԭ��Cr2O72-�����ʵ���Ϊ mol��
��2����֪�������Һ��c(Fe3+)Ϊ2.6��10��13 mol��L��1������Һ��Cr3+Ũ��Ϊ mol��L��1��
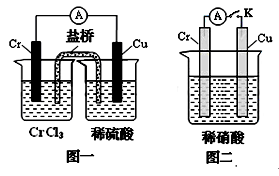
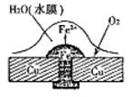
��3������ͼװ���У��۲쵽ͼһװ��ͭ�缫�ϲ�����������ɫ���ݣ�����ͼ��װ���е�����K�Ͽ�ʱ�����缫������K�պ�ʱ�����缫�ϲ���������ɫ���壬����ɺ���ɫ���塣���������������Ʋ��������������Ҫ��ѧ���� �� �� ��
����֪��������Ksp Fe(OH)3=2.6��10��39��Ksp Cr(OH)3=6.0��10��31��

��1������������������16.8g���������ϱ���ԭ��Cr2O72-�����ʵ���Ϊ mol��
��2����֪�������Һ��c(Fe3+)Ϊ2.6��10��13 mol��L��1������Һ��Cr3+Ũ��Ϊ mol��L��1��
��3������ͼװ���У��۲쵽ͼһװ��ͭ�缫�ϲ�����������ɫ���ݣ�����ͼ��װ���е�����K�Ͽ�ʱ�����缫������K�պ�ʱ�����缫�ϲ���������ɫ���壬����ɺ���ɫ���塣���������������Ʋ��������������Ҫ��ѧ���� �� �� ��
��0.05 ��2�֣�
��6.0��10��5��2�֣�
�Ǹ���ͭ���ã�����ϡ�����п��Է����ۻ���2�֣���1�֣�
��6.0��10��5��2�֣�
�Ǹ���ͭ���ã�����ϡ�����п��Է����ۻ���2�֣���1�֣�
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
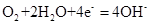
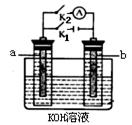
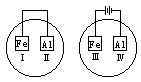
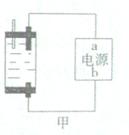
 ��2��ͼ2��a��b�Ƕ��ʯī�缫���Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��ֻ�������ڶ������ݽ��缫��Χ��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���Ȼ��Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���
��2��ͼ2��a��b�Ƕ��ʯī�缫���Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��ֻ�������ڶ������ݽ��缫��Χ��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���Ȼ��Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���