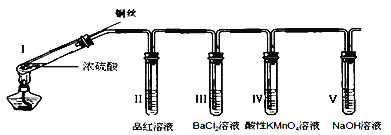
��Ŀ����
����Ŀ����������Cu2O���Ǻ�ĸ��ͧ�ײ��ķ���ʴͿ�ϣ�Ҳ�������Ĵ�����
(1)��֪��C��s��+![]() O2��g��=CO��g����H ���C110.4kJmol-1��
O2��g��=CO��g����H ���C110.4kJmol-1��
2Cu2O��s��+O2��g���� 4CuO��s�� ��H ���C292kJmol-1����ҵ����̼����CuO��ĩ�����һ�������·�Ӧ��ȡCu2O(s)��ͬʱ����CO������Ȼ�ѧ����ʽΪ________��
(2)������Cu2O��������ʵ�ּ״�������ȡ��ȩ��
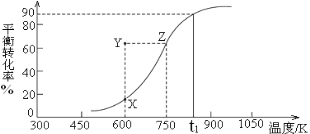
CH3OH(g)![]() HCHO(g)+H2(g),�״���ƽ��ת�������¶ȱ仯��������ͼ��ʾ��
HCHO(g)+H2(g),�״���ƽ��ת�������¶ȱ仯��������ͼ��ʾ��
���÷�Ӧ����H___0 ������>������<������600Kʱ��Y��״���v(��) ____v(��)������>������<������
����Y�㵽X��ɲ�ȡ�Ĵ�ʩ��___________________________________��
����t1Kʱ����̶����Ϊ2L���ܱ������г���1molCH3OH(g)���¶ȱ��ֲ��䣬9����ʱ�ﵽƽ��,��0��9min����CH3OH(g)��ʾ�ķ�Ӧ����v(CH3OH)��_____________, �¶�Ϊt1ʱ���÷�Ӧ��ƽ�ⳣ��K��____________��
��.����ͭ����ǿ��Ӧ�ù㷺��
�ɻ�ͭ��ұ���õ��Ĵ�ͭ������⾫�����ܵõ���ͭ�����ʱ����ͭ��______���������ĵ缫��ӦʽΪ_______________��
��.��ͭ���ӵĵķ�ˮ�������Ⱦ��ͨ������ת��Ϊ��ͭ��������ȥ��
��֪��Ksp[CuS]=1��10-36��Ҫʹͭ���ӵ�Ũ�ȷ����ŷű���������0.5mg/L������Һ�е������ӵ����ʵ���Ũ������Ϊ__________mol/L��������С�����һλ����
���𰸡�2CuO��s��+C��s��=CO��g��+Cu2O��s������H =+35.6kJmol-1 > < ��С�������ѹǿ 0.05 mol��L��1��min��1 �� 4.05 Cu2++2e-=Cu 1.3��10-3 1mol/L
��������
������1�� ��C��s��+![]() O2��g��=CO��g����H ���C110.4kJmol-1��
O2��g��=CO��g����H ���C110.4kJmol-1��
��2Cu2O��s��+O2��g���� 4CuO��s�� ��H ���C292kJmol-1
���ݸ�˹����1/2��[�١�2-��]�ɵ���2CuO��s��+C��s��=CO��g��+Cu2O��s������H =1/2��[-110.4��2+292]= +35.6kJmol-1�����Ը÷�Ӧ���Ȼ�ѧ����ʽ��2CuO��s��+C��s��=CO��g��+Cu2O��s������H =+35.6kJmol-1 ���������������������2CuO��s��+C��s��=CO��g��+Cu2O��s������H =+35.6kJmol-1 ��
(2) �ٸ���ͼ�������¶ȣ��״���ƽ��ת��������˵��ƽ�������ƶ�������ӦΪ���ȷ�Ӧ����H��0��600Kʱ���״���ת����С��X����Y�㷴Ӧ������У���(��)����(��)������������������ǣ���������
��Ҫ��С�״���ת���ʣ�����ͨ����С�������ѹǿʹƽ�������ƶ�������ʵ�ִ�Y�㵽X��仯������������������ǣ���С�������ѹǿ��
����t1Kʱ���״���ƽ��ת����Ϊ90%����̶����Ϊ2L���ܱ������г���1molCH3OH(g)���¶ȱ��ֲ��䣬9����ʱ�ﵽƽ�⣬ƽ��ʱ���״������ʵ���Ϊ0.1mol�����ȩΪ0.9mol������Ϊ0.9mol������Ũ�ȷֱ�Ϊ0.05mol/L��0.45mol/L��0.45mol/L����0��9min����CH3OH(g)��ʾ�ķ�Ӧ����v(CH3OH)����0.5-0.05��/9=0.05 mol��L��1��min��1��t1Kʱ���÷�Ӧ��ƽ�ⳣ��K��0.45��0.45/0.05=4.05 mol/L��
����������������ǣ�0.05 mol��L��1��min��1��4.05 mol��L��1��
��.��ͭ������⾫�����ܵõ���ͭ�����ʱ����ͭ��������ͭ�����������õ���������ͭ�������ĵ缫��ӦʽΪ��Cu2++2e-=Cu������������������ǣ�����Cu2++2e-=Cu��
��. Ksp[CuS]=c(Cu2+)c(S2-)=1��10-36��ͭ���ӵ�Ũ��Ϊ0.5mg/L=5��10-4g/L=(5��10-4)/64mol/L������c(S2-)= Ksp[CuS]/c(Cu2+)=1��10-36/[(5��10-4)/64]��1.3��10-31mol/L;�������������������1.3��10-31mol/L��