��Ŀ����
����Ŀ��ɫͪ�����K���п�������Ѫ֬���������ԣ���ϳ�·�����£�

��֪��
��1��A�Ľṹ��ʽ��_________������ϵͳ��������F��������__________��
��2��B��C�����Լ�a��__________���Լ�b�Ľṹ��ʽ��_________��
��3��C��������NaOH��Ӧ�Ļ�ѧ����ʽΪ__________��
��4��G������Cu(OH)2��Ӧ�Ļ�ѧ����ʽΪ__________��
��5����֪����2H![]() J+H2O����J�ĺ˴Ź�������ֻ������塣��E��JΪԭ�Ϻϳ�K��Ϊ������Ӧ��д���йػ�����Ľṹ��ʽ��________��
J+H2O����J�ĺ˴Ź�������ֻ������塣��E��JΪԭ�Ϻϳ�K��Ϊ������Ӧ��д���йػ�����Ľṹ��ʽ��________��

���𰸡� ![]() 1-���� Br2��Fe����FeBr3��
1-���� Br2��Fe����FeBr3�� 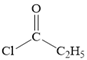
![]() CH3CH2CHO + 2Cu(OH)2 + NaOH CH3CH2COONa + Cu2O + 3H2O E��
CH3CH2CHO + 2Cu(OH)2 + NaOH CH3CH2COONa + Cu2O + 3H2O E�� ��J��
��J��![]() ������1��
������1��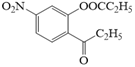 ������2��
������2��
��������A(��)����������Ӧ����B��BΪ������������C�Ļ�ѧʽ��֪���Լ�aΪ�壬����������ʱ��B���巢�������ϵ�ȡ����Ӧ����C�����D�Ľṹ��֪CΪ![]() ��������Ϣ����D��AlCl3����ʱ���Լ�b��Ӧ����E������E�Ļ�ѧʽ��֪���Լ�bΪ
��������Ϣ����D��AlCl3����ʱ���Լ�b��Ӧ����E������E�Ļ�ѧʽ��֪���Լ�bΪ ����EΪ
����EΪ ��G�ܹ���������ͭ��Ӧ����GΪȩ�����FΪ����HΪ�ᣬ���FΪCH3CH2CH2OH��GΪCH3CH2CHO��HΪCH3CH2COOH��
��G�ܹ���������ͭ��Ӧ����GΪȩ�����FΪ����HΪ�ᣬ���FΪCH3CH2CH2OH��GΪCH3CH2CHO��HΪCH3CH2COOH��
(1)��������������AΪ����F ΪCH3CH2CH2OH������Ϊ1-�������ʴ�Ϊ��![]() ��1-������
��1-������
(2)B���巢��ȡ����Ӧ����C����Ӧ��Ҫ�����������Լ�a��Br2��Fe���Լ�bΪ ���ʴ�Ϊ��Br2��Fe(��FeBr3)��
���ʴ�Ϊ��Br2��Fe(��FeBr3)�� ��
��
(3)CΪ![]() ����������NaOH��Ӧ�Ļ�ѧ����ʽΪ
����������NaOH��Ӧ�Ļ�ѧ����ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(4)GΪCH3CH2CHO��G������Cu(OH)2��Ӧ�Ļ�ѧ����ʽΪCH3CH2CHO + 2Cu(OH)2 + NaOH CH3CH2COONa + Cu2O + 3H2O���ʴ�Ϊ��CH3CH2CHO + 2Cu(OH)2 + NaOH CH3CH2COONa + Cu2O + 3H2O��
(5)HΪCH3CH2COOH����2H![]() J+H2O����J
J+H2O����J![]() ��EΪ
��EΪ ��JΪ
��JΪ![]() ��������Ϣ�ڣ��м����1Ϊ
��������Ϣ�ڣ��м����1Ϊ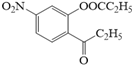 ��������Ϣ�ۣ��м����2Ϊ
��������Ϣ�ۣ��м����2Ϊ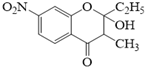 ��
�� �����ǻ�����ȥ��Ӧ����K(
�����ǻ�����ȥ��Ӧ����K( )���ʴ�Ϊ��E��
)���ʴ�Ϊ��E�� ��J��
��J��![]() ������1��
������1�� ������2��
������2�� ��
��