��Ŀ����
����Ŀ��ij��ɫ��ˮ�п��ܺ���NH4+��Fe3+��Al3+��Na+��NO3-��CO32-��SO42-�еļ��֣�Ϊ������ɷ֣���������������ʵ�飺
��ȡ��Һ����ɫ��Ӧ������Ϊ��ɫ
��ȡ��ˮ��Ʒl00mL�����μ�������BaCl2��Һ��ϡHC1���ð�ɫ����23.3g
����ȡ��ˮ��Ʒl00mL�����м���Na2O2���壬�����ij���������������Na2O2�������ʵ����Ĺ�ϵ������ͼ

��ش��������⣺
��1����������3��ʵ����Է�����ˮ��һ�������ڵ������У�________________��
��2��д��ͼ���г����ܽ�η��������ӷ�Ӧ����ʽ��_________________��____________________��
��3������ͼ�������֪����ԭ��Һ��һ�����ڵ������Ӽ������ʵ�����ֵΪ____________��
��4�� NO3-�Ƿ���ڣ�_____________�� (����ڡ������ڡ���ȷ�����������������ʵ���Ũ��Ϊ_________________�����������ڻ�ȷ�����ÿղ��
���𰸡� Fe3+��CO32- 2Na2O2+2H2O=4Na++4OH-+O2�� Al(OH)3+OH-=AlO2-+2H2O n(NH4+)��n(Al3+)=2��1 ���� c(NO3-)>3mol/L
����������ȡ��Һ����ɫ��Ӧ������Ϊ��ɫ��˵����Һ��һ����Na+����ȡ��ˮ��Ʒl00mL�����μ�������BaCl2��Һ��ϡHC1���ð�ɫ����23.3g���ó���������ϡ���ᣬ˵���ó��������ᱵ�������ʵ���Ϊ0.1mol����ԭ100mL��Һ����0.1mol SO42-������ȡ��ˮ��Ʒl00mL�����м���Na2O2���壬�����ij���������������Na2O2�������ʵ����Ĺ�ϵ������ͼ����ͼ���֪����Һ��һ����NH4+������0.3mol Na2O2�����������Ͱ����Ļ�����干0.35mol����0.3mol Na2O2��ˮ��Ӧ������0.15mol������0.6mol�������ƣ��������ɵİ�����0.2mol��ԭ100mL��Һ����0.2mol NH4+��������������ɵij������ܽ��ˣ����Ըó�������������������ԭ��Һ��һ����Al3+����Fe3+�����������������������ƣ���CO32-����Al3+���ܴ������棩��ԭ100mL��Һ����0.1mol Al3+��
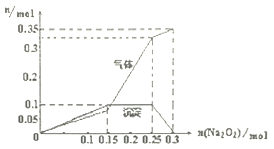
��1����������3��ʵ����Է�����ˮ��һ�������ڵ������У�Fe3+��CO32-��
��2��ͼ���г����ܽ�η��������ӷ�Ӧ����ʽΪ2Na2O2+2H2O=4Na++4OH-+O2�� ��Al(OH)3+OH-=AlO2-+2H2O��
��3������ͼ�������֪����ԭ��Һ��һ�����ڵ������Ӽ������ʵ�����ֵΪn(NH4+)��n(Al3+)=2��1��
��4�����ݵ���غ�����жϣ�NO3-һ�����ڡ���100mL��Һ����n(NO3-)= n(NH4+)+3n(Al3+)+n(Na+)-2n(SO42-)= n(Na+)+0.3mol��n(NO3-)>0.3mol�����������ʵ���Ũ��c(NO3-)>3mol/L��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��NAΪ�����ӵ�������ֵ�����ı������ݣ������ж��ڱ�״������ȷ����
NO2 | HF | |
�۵�/�� | -11.2 | -83.6 |
�е�/�� | 21.0 | 19.52 |
A. 2.0gHF�к�������ĿΪl.0NA
B. 12gʯīϩ(����ʯī)�к�����Ԫ������ΪNA
C. 6.72LNO2��ˮ��Ӧ��ת�Ƶĵ�����Ϊ0.2NA
D. NO��O2��2.24L��ַ�Ӧ�����û������ϵ�к�������Ϊ0.1NA