��Ŀ����
ȼ�շ��Dzⶨ�л������ﻯѧʽ��һ����Ҫ��������һ���¶���ȡ0.1molijҺ̬��A��O2����ȫȼ�գ�����CO2��ˮ������������������ͨ��Ũ����ͼ�ʯ�ң�Ũ��������16.2g����ʯ������35.2g��
��1��д����A�ķ���ʽ
��2��������������Aͬ���칹�����ĿΪ
��3����A��һ��ͬ���칹�壬��һ�ȴ���ֻ��һ�֣�д����ͬ���칹��Ľṹ��ʽ
��1��д����A�ķ���ʽ
C8H18
C8H18
����2��������������Aͬ���칹�����ĿΪ
4
4
����3����A��һ��ͬ���칹�壬��һ�ȴ���ֻ��һ�֣�д����ͬ���칹��Ľṹ��ʽ
��CH3��3CC��CH3��3
��CH3��3CC��CH3��3
����������1��Ũ��������16.2gΪ���ɵ�ˮ������������Hԭ���غ�����0.1molijҺ̬��A��Hԭ�ӵ����ʵ�������ʯ������35.2gΪȼ������CO2������������Cԭ���غ�����0.1molijҺ̬��A��Cԭ�ӵ����ʵ������Դ˿�������ķ���ʽ��
��2��C8H18��������������������˵�����������1��֧������������֧��������Cԭ����7��6������֧����Ӧ��Cԭ�ӷֱ���1��2����
��3��һ�ȴ���ֻ��һ�֣��������ֻ��һ��Hԭ�ӣ��ݴ˽��
��2��C8H18��������������������˵�����������1��֧������������֧��������Cԭ����7��6������֧����Ӧ��Cԭ�ӷֱ���1��2����
��3��һ�ȴ���ֻ��һ�֣��������ֻ��һ��Hԭ�ӣ��ݴ˽��
����⣺��1��n��H2O��=
=0.9mol
����0.1molijҺ̬��A��Hԭ�����ʵ���Ϊn��H��=0.9mol��2=1.8mol
n��CO2��=
0.8mol
����0.1molijҺ̬��A��Hԭ�����ʵ���Ϊn��C��=0.8mol
����A�ķ���ʽΪC8H18��
�ʴ�Ϊ��C8H18
��2������Cԭ����7����֧����Ӧ��Cԭ����1������������ͬ���칹�������֣��ֱ�Ϊ
CH3CH��CH3��CH2CH2CH2CH2CH3��CH3CH2CH��CH3��CH2CH2CH2CH3��CH3CH2CH2CH��CH3��CH2CH2CH3
����Cԭ����6������֧����Ӧ��Cԭ����2������������ͬ���칹����һ��ΪCH3CH2CH��C2H5��CH2CH2CH3
�ʴ�Ϊ��4
��3��������ֻ��һ��Hԭ�ӣ�C8H18������������Hԭ��һ��λ�ڼ��ϣ����Լ���ĿΪ
=6��ʣ���2��Cԭ��ͨ���������ӣ�ÿ��Cԭ������3�������ṹ��ʽΪ��CH3��3CC��CH3��3��
�ʴ�Ϊ����CH3��3CC��CH3��3
| 16.2g |
| 18g/mol |
����0.1molijҺ̬��A��Hԭ�����ʵ���Ϊn��H��=0.9mol��2=1.8mol
n��CO2��=
| 35.2 |
| 44g/mol |
����0.1molijҺ̬��A��Hԭ�����ʵ���Ϊn��C��=0.8mol
����A�ķ���ʽΪC8H18��
�ʴ�Ϊ��C8H18
��2������Cԭ����7����֧����Ӧ��Cԭ����1������������ͬ���칹�������֣��ֱ�Ϊ
CH3CH��CH3��CH2CH2CH2CH2CH3��CH3CH2CH��CH3��CH2CH2CH2CH3��CH3CH2CH2CH��CH3��CH2CH2CH3
����Cԭ����6������֧����Ӧ��Cԭ����2������������ͬ���칹����һ��ΪCH3CH2CH��C2H5��CH2CH2CH3
�ʴ�Ϊ��4
��3��������ֻ��һ��Hԭ�ӣ�C8H18������������Hԭ��һ��λ�ڼ��ϣ����Լ���ĿΪ
| 18 |
| 3 |
�ʴ�Ϊ����CH3��3CC��CH3��3
���������⿼���л������ʽ��ȷ������������ͬ���칹�����д����Ŀ�еȣ�ע�����������Ϣ��

��ϰ��ϵ�д�
�����Ŀ
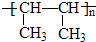
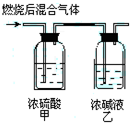 ȼ�շ��Dzⶨ�л������ﻯѧʽ��һ����Ҫ������������ȫȼ��0.1molij����ȼ�ղ�������ͨ����ͼ��ʾ��װ�ã�ʵ������Ƶü�װ������10.8g����װ������22g��������Ļ�ѧʽ����д�������еĽṹ��ʽ��
ȼ�շ��Dzⶨ�л������ﻯѧʽ��һ����Ҫ������������ȫȼ��0.1molij����ȼ�ղ�������ͨ����ͼ��ʾ��װ�ã�ʵ������Ƶü�װ������10.8g����װ������22g��������Ļ�ѧʽ����д�������еĽṹ��ʽ��