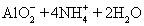МвДүДЪИЭ
КµСйКТАпіӘУГµДёЙФпәБУРӘғұЩЕЁБтЛбӘЁ98%Ә©Ә¬ұЪОЮЛ®ВИ»ҮёЖӘ¬ұЫ±дЙ«№иҢғӘЫ№иҢғµДЦчТҒіЙ·ЦКЗ¶юСх»Ү№иӘ¬ФЪЖдЦРІфИлЙЩБүµДОЮЛ®ВИ»ҮоЬӘЁCoCl2Ә©ЧчЦёКңәБӘ¬ОЮЛ®ВИ»ҮоЬіКА¶Й«Ә¬ОьЛ®ғу±дОҒCoCl2Ұ¤6H2OіК·ЫғмЙ«ӘЭӘ¬ұЬОеСх»Ү¶юБЧӘ¬ұЭәоКҮ»ТӘЁЦчТҒіЙ·ЦКЗЗвСх»ҮДЖҰұСх»ҮёЖӘ¬ЦЖ·ЁКЗӘғ°СЙъКҮ»ТәУµҢЕЁµДЙХәоИЬТғЦРӘ¬ФЩәУЗүИИХфёЙӘ©µИҰӘ
ӘЁ1Ә©РөіцЦЖИҰёЙФпәБәоКҮ»Т№эіМЦРУР№Ш·өУ¦µД»ҮС§·ҢіМКҢӘғ__________________________________ҰӘ
ӘЁ2Ә©ЙПКцОпЦКЦРӘ¬КфУЪөүң»ОпµДКЗ______ҰӘ
AӘ®ұЩұЪұЬ BӘ®ұЪұЬ
CӘ®ұЩұЪұЬұЭ DӘ®И«Іү
ӘЁ3Ә©ЙПКцёЙФпәБЦРӘ¬І»ТЛУГУЪёЙФпВИ»ҮЗвЖшМеµДКЗ______ҰӘ
ӘЁ4Ә©ЙПКцұЩҰ«ұЬӘ¬ЖдЦчТҒ»ҮС§іЙ·ЦТАөОКфУЪ______Ұұ______Ұұ______Ұұ______ ӘЁМоРөёчОпЦКЛщКфµДАа±рӘ©ҰӘ
ӘЁ1Ә©CaOӘ«H2O=CaӘЁOHӘ©2ҰұCaӘЁOHӘ©2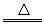 CaOӘ«H2O
CaOӘ«H2O
ӘЁ2Ә©B ӘЁ3Ә©ұЭ ӘЁ4Ә©Лб СО Сх»ҮОп Сх»ҮОп
ҰңҢвОцҰүЕЁБтЛбКЗH2SO4УлЛ®µД»мғПОпӘ¬ФЪОпЦК·ЦАаЦРКфУЪЛбӘ»ОЮЛ®ВИ»ҮёЖКЗөүң»ОпӘ»±дЙ«№иҢғКЗSiO2ғНCoCl2µД»мғПОпӘ»P2O5КЗөүң»ОпӘ»әоКҮ»ТКЗNaOHҰұCaOµД»мғПОпҰӘ

 КЦАКЦИ«УЕБ·үәңнПµБРөр°ё
КЦАКЦИ«УЕБ·үәңнПµБРөр°ё