��Ŀ����
��10�֣����������Ǽ��Զ��ε�ص��������ϡ����ظ���ˮ���Ǻϳ�Ni (OH )2����֮һ��������ˮ������İ�������Ni (OH )2 �Ľṹ�С�Ni(OH )2 �а������ߵ�������Ni (OH )2 �绯ѧ���Ե���Ҫ���ݡ�ijʵ��С��ⶨNi(OH )2�а�������ʵ�鲽�����£�
����������Ni (OH ) 2 �ֱ�����ˮ�����ᣬ�ټ���40%����NaOH ��Һ�������������еİ�������15 mL 0 .1 mol��L-1 H2SO4��Һ���ա�
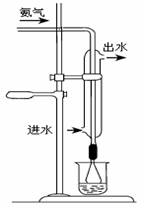 �ڵζ������ձ�����������Һȫ��ת����ƿ������ƿ�м�������ˮ����ϡ�Ͳ�����1��2�μ���һ�Ǽ������ָʾ������ɫ��Χ5.2��5.6������NaOH����Һ�ζ�ʣ������ᣬ����Һ��ɫ�ɺ���ɫתΪ��ɫʱ����Ϊ�ζ��յ㡣
�ڵζ������ձ�����������Һȫ��ת����ƿ������ƿ�м�������ˮ����ϡ�Ͳ�����1��2�μ���һ�Ǽ������ָʾ������ɫ��Χ5.2��5.6������NaOH����Һ�ζ�ʣ������ᣬ����Һ��ɫ�ɺ���ɫתΪ��ɫʱ����Ϊ�ζ��յ㡣
��������֪ʶ�ش��������⣺
��1�����հ�ʱ��Ӧ�Ļ�ѧ����ʽΪ ��
��2����������װ������ͼ��ʾ���ձ��е���©����������
��
��3���ζ�����ʹ��ǰ��ϴ���⣬��Ӧ ��
��ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ���������Ʒ�а��ĺ���
(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
��4��ȷ��ȡ 0.5 g Ni(OH ) 2��Ʒ��������������Ũ��Ϊ 0.1000 mol��L-1 �� NaOH ����Һ�ζ����յ�ʱ������20.00 mL�������Ʒ�а��ĺ���Ϊ ��
��10�֣�ÿ��2�֣�(1)2NH3 + H2SO4 = (NH4)2SO4 (2)������
(3)��© ƫ�� (4)3.4%

| �ŵ� |
| ��� |
| A���ŵ�ʱ�����ĵ缫��ӦΪMo3S4+2xe-=Mo3S42X- |
| B�����ʱ�����ĵ缫��ӦΪxMg2++2xe-=xMg |
| C���ŵ�ʱMg2+�������ƶ� |
| D���ŵ�ʱMo3S4����������Ӧ |
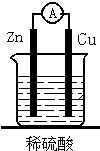 ��ͼ��һ���Ե��--ͭп��صļ���װ�ã�
��ͼ��һ���Ե��--ͭп��صļ���װ�ã�