��Ŀ����
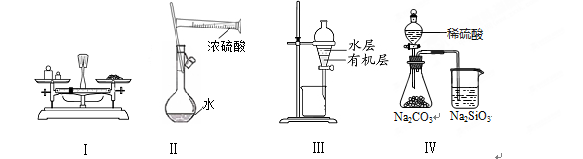
ʵ��������500mL 0.1mol/L��NaOH��Һ�������²������裺
�ټ�������NaOH�������������������ƽ��ȡ��
�ڽ������õ�NaOH��������ձ��У���������������ˮ�ܽ⣬Ȼ��ת��������ƿ�У�
������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ת������ƿ�в�����ҡ�ȣ�
�ܼ���������ƿ�м�����ˮ��Һ���̶���1��2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ����̶������У�
����������ƿ�����ӣ����ҡ�ȡ�
�ش��������⣺
��1������ƿ��ʹ��ǰ������
��2��ʵ������������ƽʵ�ʳ�ȡNaOH�����������
��3������ʵ��������У�ȱ�ٵIJ�����
��4����ʵ���У�δ���в����ۣ�������Һ��Ũ�Ȼ� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����ͬ��������ʱ����Һ�棬������Һ��Ũ�Ȼ� ������ǰ����ƿ������ˮ��������Һ��Ũ�Ȼ� ��
�ټ�������NaOH�������������������ƽ��ȡ��
�ڽ������õ�NaOH��������ձ��У���������������ˮ�ܽ⣬Ȼ��ת��������ƿ�У�
������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ת������ƿ�в�����ҡ�ȣ�
�ܼ���������ƿ�м�����ˮ��Һ���̶���1��2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ����̶������У�
����������ƿ�����ӣ����ҡ�ȡ�
�ش��������⣺
��1������ƿ��ʹ��ǰ������
��2��ʵ������������ƽʵ�ʳ�ȡNaOH�����������
��3������ʵ��������У�ȱ�ٵIJ�����
��4����ʵ���У�δ���в����ۣ�������Һ��Ũ�Ȼ� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����ͬ��������ʱ����Һ�棬������Һ��Ũ�Ȼ� ������ǰ����ƿ������ˮ��������Һ��Ũ�Ȼ� ��
��1�������Ƿ�©ˮ ��2��2.0g ��3����ȴ ��4��ƫ�� , ƫ�� , ��Ӱ��
�����������1������ƿ��ȷ����һ�������һ��Ũ�ȵ���Һ���������������Ƶ���Һ�ɾ�һ�ԡ��ȶ��ԡ������Ƶ����һ��Ҫҡ�ȡ�������ʹ��ǰ������������ƿ�Ƿ�©ˮ����2��n(NaOH)="C��V=" 0.1mol/L��0.5L="0.05mol.m(NaOH)=" 0.05mol��40g/mol=2.0g.������ƽ��ȷ��Ϊ0.1g,����ʵ������������ƽʵ�ʳ�ȡNaOH�����������2.0g. ��3�� NaOH�����ܽ���ˮ�зų�������������ƿ������ҺʱҪ����¶�������20�ȣ���������ʵ��������У�ȱ�ٵIJ�������ȴ�����¡���4����ʵ���У��ܽ�����ʹ�õ��ձ����ڱڼ��������϶�մ�����ʣ���δ���в����ۣ��ͻ�ʹһ��������û����ȫת�Ƶ�����ƿ�У�����������Һ��Ũ�Ȼ�ƫ�͡�����ʱ�������Һ�棬��������Һ������ͻ�ƫС����������Һ��Ũ�Ȼ�ƫ�ߡ��������ǰ����ƿ������ˮ��������δ�ı����ʵ����ʵ����Ķ��٣�ҲδӰ����Һ������Ĵ�С�����Զ�������Һ��Ũ�Ȳ�������κ�Ӱ�졣

��ϰ��ϵ�д�
�����Ŀ