��Ŀ����
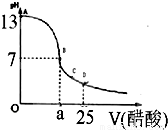
 ijѧ����ʵ���Ҳⶨһδ֪Ũ�ȵ�ϡ���ᣬ��֪��25ml�������Ʊ���Һ����μ���0.2mol/L������Һ��PH�仯������ͼ��ʾ��
ijѧ����ʵ���Ҳⶨһδ֪Ũ�ȵ�ϡ���ᣬ��֪��25ml�������Ʊ���Һ����μ���0.2mol/L������Һ��PH�仯������ͼ��ʾ����1��������������Һ�����ʵ���Ũ��Ϊ
��2����B�㣬a
��3������100mL NaOH����Һ����������������ƽ������������ͷ�ι��⣬����Ҫ
��4����
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 19.00 | 23.00 | 23.02 |
�ζ��ﵽ�յ�ı�־��
��2��B��pH=7����Ϊǿ��ʱ��Ũ��Ϊ2����ϵ�������Ϊһ�룬����Ϊ���
��3������100mLNaOH����Һ������ʹ��100mL����ƿ���ձ���������ƽ������������ͷ�ιܵȣ�
��4��������ʽ�ζ���ʢ�ᣬ������̪����ɫ��������̪��죬����c���=
| c(��)V(��) |
| V(��) |
��2��B��pH=7����Ϊǿ��ʱ����Ũ��Ϊ��Ũ�ȵ�2���������Ϊһ�룬����Ϊ���ᣬǡ�÷�ӦʱΪ���ԣ����������������12.5mL���ʴ�Ϊ������
��3������100mLNaOH����Һ������ʹ��100mL����ƿ���ձ���������ƽ������������ͷ�ιܵȣ�
�ʴ�Ϊ��100mL����ƿ���ձ���
��4��������ʽ�ζ�����ȡ20.00mL����ϡ������Һ������ƿ�У���������̪����ɫ��������̪��죬��ζ��յ�ı�־Ϊ���һ��NaOH��Һ���룬��Һ����ɫǡ�ñ��dz��ɫ��������ڲ���ɫ����c���=
| c(��)V(��) |
| V(��) |
| 23.00+23.02 |
| 2 |
| 0.1mol/L��0.02301L |
| 0.020L |
�ʴ�Ϊ����ʽ�ζ��ܣ�0.12mol/L�����һ��NaOH��Һ���룬��Һ����ɫǡ�ñ��dz��ɫ��������ڲ���ɫ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���12�֣�ijѧ����ʵ���Ҳⶨһδ֪Ũ�ȵ�ϡ����,��֪��25ml�������Ʊ���Һ����μ���0.2mol/L������Һ��PH�仯������ͼ��ʾ��
��1��������������Һ�����ʵ���Ũ��Ϊ mol.L��1
��2����B�㣬a 12.5ml(�>������<����=�� )��
��3������100 mL NaOH����Һ����������������ƽ������������ͷ�ι��⣬����Ҫ
��4���� ��ȡ20.00 mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ������NaOH����Һ���еζ���Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡϡ���������Ϊ20.00 mL������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 19.00 | 23.00 | 23.02 |
�������Ũ��ԼΪ___________________(������λ��Ч����)��
�ζ��ﵽ�յ�ı�־��
��12�֣�ijѧ����ʵ���Ҳⶨһδ֪Ũ�ȵ�ϡ����,��֪��25ml�������Ʊ���Һ����μ���0.2mol/L������Һ��PH�仯������ͼ��ʾ��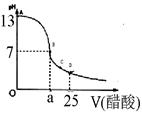
��1��������������Һ�����ʵ���Ũ��Ϊ mol.L��1
��2����B�㣬a 12.5ml(�>������<����="��" )��
��3������100 mL NaOH����Һ����������������ƽ������������ͷ�ι��⣬����Ҫ
��4���� ��ȡ20.00 mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ������NaOH����Һ���еζ���Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡϡ���������Ϊ20.00 mL������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 19.00 | 23.00 | 23.02 |
�ζ��ﵽ�յ�ı�־��
��12�֣�ijѧ����ʵ���Ҳⶨһδ֪Ũ�ȵ�ϡ����,��֪��25ml�������Ʊ���Һ����μ���0.2mol/L������Һ��PH�仯������ͼ��ʾ��
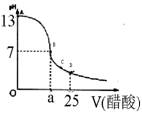
��1��������������Һ�����ʵ���Ũ��Ϊ mol.L��1
��2����B�㣬a 12.5ml(�>������<����=�� )��
��3������100 mL NaOH����Һ����������������ƽ������������ͷ�ι��⣬����Ҫ
��4���� ��ȡ20.00 mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ������NaOH����Һ���еζ���Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡϡ���������Ϊ20.00 mL������ʵ������¼���£�
|
ʵ����� |
��һ�� |
�ڶ��� |
������ |
|
����NaOH��Һ���/mL |
19.00 |
23.00 |
23.02 |
�������Ũ��ԼΪ___________________ (������λ��Ч����)��
�ζ��ﵽ�յ�ı�־��
��1��������������Һ�����ʵ���Ũ��Ϊ______ mol��L-1
��2����B�㣬a______12.5ml�����������������=������
��3������100mL NaOH����Һ����������������ƽ������������ͷ�ι��⣬����Ҫ______
��4����______��ȡ20.00mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ������NaOH����Һ���еζ���Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡϡ���������Ϊ20.00mL������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 19.00 | 23.00 | 23.02 |
�ζ��ﵽ�յ�ı�־��______��