��Ŀ����
���ڹ�ũҵ������Ӧ�ù㷺��
��1���� ����ͼд���ϳɰ����Ȼ�ѧ����ʽ��________________________��
����ͼд���ϳɰ����Ȼ�ѧ����ʽ��________________________��

��2����1 mol N2(g)��3 mol H2(g)����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų�������___________(����ڡ��������ڡ���С�ڡ�)92.2 kJ��ԭ����______________���������������H________(���������䡱��С��)��
��3����֪�ֱ��ƻ�1 mol N��N����1 mol H��H��ʱ��Ҫ���յ�����Ϊ946 kJ��4 36 kJ�����ƻ�1 mol N��H����Ҫ���յ�����Ϊ________kJ��
36 kJ�����ƻ�1 mol N��H����Ҫ���յ�����Ϊ________kJ��
��4��N2H4����ΪNH3�����е�H����NH2ȡ���IJ����������ʱ��N2H4(g)Ϊȼ�ϡ�NO2Ϊ�����������߷�Ӧ����N2��H2O(g)��
��֪��N2(g)��2O2(g)==2NO2(g) ��H1����67.7 kJ��mol��1
N2H4(g)�� O2(g)==N2(g)��2H2O(g) ��H2����534 kJ��mol��1
O2(g)==N2(g)��2H2O(g) ��H2����534 kJ��mol��1
��1 mol N2H4��NO2��ȫ��Ӧ���Ȼ�ѧ����ʽΪ_______________��
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д��屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£�
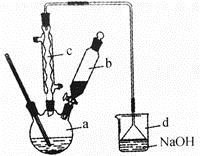
| �� | �� | �屽 |
�ܶ�/g�qcm-3 | 0.88 | 3.10 | 1.50 |
�е�/�� | 80 | 59 | 156 |
ˮ���ܽ�� | �� | �� | �� |
�����кϳɲ���ش����⣺
��1����a�м���15mL��ˮ����������м����b��С�ļ���4.0mLҺ̬�壬��a�е��뼸���壬�а�ɫ��������������Ϊ������ ���塣�����μ���Һ����꣬װ��d�������� ��
��2��Һ�����������в�������ᴿ��
����a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����Һ������10mLˮ��8mL10%��NaOH��Һ��10mLˮϴ�ӣ�NaOH��Һϴ�ӵ������� ��
��3�������Ϸ���������屽�л����е���Ҫ����Ϊ ��Ҫ��һ���ᴿ�����в����б���Ҫ������ ��������ȷ����ǰ����ĸ��
A���ؽᾧ B������ C������ D����ȡ


 H����A2�����ش��������⣺
H����A2�����ش��������⣺ H+ +H2PO3����������������NaOH��Һ��Ӧ������Na2HPO3��
H+ +H2PO3����������������NaOH��Һ��Ӧ������Na2HPO3�� CH2==CH2(g)+4H2O(g) ��H=a kJ��mol-1 ��֪��H2(g)��ȼ����Ϊ285.8 kJ��mol-1��CH2=CH2(g)��ȼ����Ϊ1411.0 kJ��mol-1��H2O(g)= H2O(l) ��H=-44.0 kJ��mol-1����a=______kJ��mol-1��
CH2==CH2(g)+4H2O(g) ��H=a kJ��mol-1 ��֪��H2(g)��ȼ����Ϊ285.8 kJ��mol-1��CH2=CH2(g)��ȼ����Ϊ1411.0 kJ��mol-1��H2O(g)= H2O(l) ��H=-44.0 kJ��mol-1����a=______kJ��mol-1��
 2CO2(g)+N2(g)��
2CO2(g)+N2(g)�� =1 ��NO����ʼŨ��Ϊamol/L,NO��ת����Ϊ80��������¶�ʱ��ƽ�ⳣ��K=________��
=1 ��NO����ʼŨ��Ϊamol/L,NO��ת����Ϊ80��������¶�ʱ��ƽ�ⳣ��K=________��
 H����0.33 kJ��mol��1
H����0.33 kJ��mol��1
 Ϊƫ������Ψһ��һ��ͬ���칹��
Ϊƫ������Ψһ��һ��ͬ���칹�� ����������������Ӧ�Ļ�ѧ����ʽΪ��(CH3)2NNH2��2N2O4��2CO2��4H2O��3N2
����������������Ӧ�Ļ�ѧ����ʽΪ��(CH3)2NNH2��2N2O4��2CO2��4H2O��3N2 ȷ����
ȷ���� ��ϵͳ�������������л������Ϊ2,2,3����������
��ϵͳ�������������л������Ϊ2,2,3����������