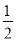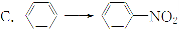��Ŀ����
Ԫ��W��X��Y��Z��M��N��Ϊ����������Ԫ�أ���ԭ����������������֪Yԭ������������������������֮��Ϊ3��4��Mԭ�ӵ����������������������֮��Ϊ3��4����Mԭ�ӵ���������Yԭ�ӵ�2����XԪ�ص��������������۾���ֵ֮��Ϊ2��N����Z����W���İ뾶��С��������WN������Ϊ���壬�ݴ˻ش��������⣺
(1)W��Y�ɷֱ��γ�10���Ӻ�18���ӵķ��ӣ�д����18���ӷ���ת����10���ӷ��ӵĻ�ѧ����ʽ��_________________________________________��
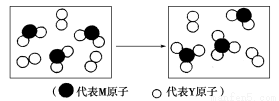
(2)��ͼ��ʾ����������Ԫ����ɵ����������һ�������µ��ܱ������г�ַ�Ӧǰ���ת����ϵ����д����ת�����̵Ļ�ѧ����ʽ��_________________��
(3)A��B��Ϊ����������Ԫ���е�����Ԫ����ɵ�ǿ����ʣ������Ԫ�ص�ԭ�Ӹ���֮�Ⱦ�Ϊ1��1��1�����ڸ��Ե�ˮ��Һ�У�A������ˮ�ĵ��룬B�ܴٽ�ˮ�ĵ��룬��A�Ļ�ѧʽΪ________��B�ĵ���ʽ��________��
(4)XY2��H2O��Ӧ�����ӷ���ʽΪ__________________________________��
��(1)2H2O2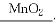 2H2O��O2��
2H2O��O2��
(2)2SO2��O2 2SO3
2SO3
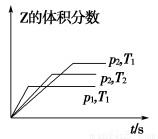
(3) 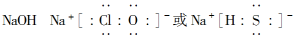
(4)3NO2��H2O=2H����2NO3����NO
����������Yԭ������������������������֮��Ϊ3��4��Mԭ�ӵ����������������������֮��Ϊ3��4����Mԭ�ӵ���������Yԭ�ӵ�2�������Ƴ�YΪO��MΪS��XԪ�ص�ԭ������С��Y��XԪ�ص��������������۾���ֵ֮��Ϊ2�����Ƴ�XΪN��N����Z����W���İ뾶��С��������WN������Ϊ���壬���Ƴ�WΪH��ZΪNa��NΪCl��W��Y�γɵ�10���ӷ���ΪH2O,18���ӷ���ΪH2O2��NaOH��NaClO��NaHS��Ϊǿ����ʣ�NaOH������ˮ�ĵ��룬NaClO��NaHS�ܴٽ�ˮ�ĵ��롣

 �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д� ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д�