��Ŀ����
��֪ú�Ľ����ṹģ����ͼ��ʾ��
�ش��������⣺
(1)��ú�Ľṹģ��������ú�ǹ�ҵ�ϻ��____________����Ҫ��Դ��
(2)�ҹ���Լ70%��ú��ֱ������ȼ�յġ���ú�Ľṹģ�����������ṩ������ͬʱ����������____________��____________���������ʣ�������صĴ�����Ⱦ��
(3)����ú�������������Լ���87%�ķ����ŷ������̳��ŷ���Ҳ�ɼ���80%���°��ﱽ��[��]�ŵ��ŷ���Ҳ���٣�ͬʱ��ú20%��30%������ú��������ԭ�������ù������ȼ�չ����������ȶ��������Ρ�ij����ú������������ʯ��ʯ������������û�ѧ����ʽ��ʾ��������________________��________________��
(4)Ϊ�˽��úȼ������ɵ���Ⱦ��������ú�����ü�ֵ��ú��Դ���ۺ����÷�������____________��____________��____________�ȡ�
(1)��������(2)SO2���������(3)CaCO3 CaO��CO2����2CaO��2SO2��O2
CaO��CO2����2CaO��2SO2��O2 2CaSO4��(4)����������Һ��
2CaSO4��(4)����������Һ��
����

ij��������200����ԭ�ӣ���ô�����ķ���ʽ��
| A��C97H200 | B��C98H200 | C��C99H200 | D��C100H200 |
��ȷ�����Ľṹ���о�������,���������нṹ:a. ����b.
����b.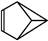 ��
��
��ش���������:
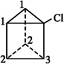
(1)�����ĽṹΪa,������ �ṹ��������Щ���治ͬ��������(����ĸ����)��
�ṹ��������Щ���治ͬ��������(����ĸ����)��
| A��һ�ȴ�������� | B�����ȴ�������� |
| C������ˮ���� | D������������ |
������Һ����ʹú��������Դ����Ч;����ú����������Ҫ��Ӧ�ǣ�C��H2O(g) CO��H2��CO��H2�Ļ�������Ǻϳɶ����л����ԭ�������о���CO��H2�ϳ��л���Ļ�ѧ��Ϊһ̼��ѧ����ͼ�Ǻϳ�ijЩ���ʵ�·��ͼ��
CO��H2��CO��H2�Ļ�������Ǻϳɶ����л����ԭ�������о���CO��H2�ϳ��л���Ļ�ѧ��Ϊһ̼��ѧ����ͼ�Ǻϳ�ijЩ���ʵ�·��ͼ��
���У�D������ˮ������CH3COOH��Ϊͬ���칹�壬F�����е�̼ԭ������D�е�3����H���������ɵõ�G����ش��������⣺
(1)д���������ʵĽṹ��ʽ��A����������������H������������������ָ��A��H�Ĺ�ϵ������������
(2)���úϳ���(H2��CO)�������͡��״��Ͱ����Ѿ�ʵ���˹�ҵ�����ϳ���Ҳ�ɺϳ�ȩ���ᡢ���ȶ��ֲ�����б�����ȷ����������������
���Ժϳ���Ϊԭ�ϵķ�Ӧ���ǻ��Ϸ�Ӧ
�ڸı�ϳ�����CO��H2������ȣ��ɵõ���ͬ�IJ���
�ۺϳ�����ת����Ӧ�����ʵ����¶Ⱥ�ѹǿ��ͨ�������
�ܴӺϳ�������������̬�������л�����ʵ�֡�ú���͡�����Ч;��
���Ժϳ���Ϊԭ�ϵķ�Ӧ�����в�������ϩ����ˮ
| A���٢ڢ� | B���ڢۢ� | C���ڢܢ� | D���ۢܢ� |
��CH3COOH��E�D��F��____________________________________________��
��D������������ͭ����Һ���ȣ�___________________________________��
ijЩ�Ͼ����Ͽɲ������з����������������ϸ���������ǿ�ȣ�ʹ�������õ����ʣ�ʵ��װ������ͼ������ij�����ϵõ��IJ������±���
| ���� | ���� | ���� | ��ϩ | ��ϩ | �� | �ױ� | ̼ |
| ��������(%) | 12 | 24 | 12 | 16 | 20 | 10 | 6 |
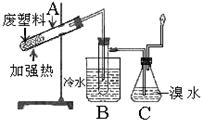
��1���Թ�B�ռ����IJ�Ʒ�У��˴Ź���������ʾ���ĸ����շ���л����������ӳɺ����ò����һ�ȴ����� �֡�
��2���Թ�A�в������ж�����;��������ת���Ϳ���ȡ�߾������Ȳ��
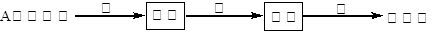
д����Ӧ�ڵĻ�ѧ����ʽ ��
��3��ZnC2��Al4C3��Mg2C3��Li2C2��Mg3N2����CaC2��H2O��Ӧ���ơ���ͨ����CaC2��C2H2�ķ�Ӧ��˼�����ж����з�Ӧ������ȷ���� ��
A��ZnC2ˮ���������飨C2H6�� B��Al4C3ˮ�����ɼ��飨CH4��
C��Mg2C3ˮ�����ɱ�Ȳ��C3H4�� D��Li2C2ˮ��������ϩ��C2H4��
E��Mg3N2ˮ�����ɰ�����NH3��
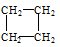 ��
��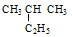 ������ ������ ��
������ ������ ��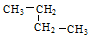 �����л�Ϊͬ���칹����� ������ţ��������֮��Ĺ�ϵΪ ��
�����л�Ϊͬ���칹����� ������ţ��������֮��Ĺ�ϵΪ ��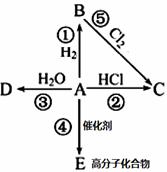
 ��CO(NH2)2
��CO(NH2)2