��Ŀ����
��1���������ż������м��أ����ࣨCuSO4��������Ϊͭ����д���÷�Ӧ�����ӷ���ʽ��
��2����ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��2Cu2O+Cu2S
6Cu+SO2������Ӧ����������
��3��������Ʒʹ�ø�Ϊ�㷺����������Ʒ�����⣬�������Ҫ�ɷ���
��4������Ʒ��Ϊ�������γ����ܵ���������Ĥ���������ã����Ǹ�����Ĥ�ױ�����ƻ�������������������������Һ���ã���Ӧ�����ӷ���ʽΪ
Fe+Cu2+�TFe2++Cu
Fe+Cu2+�TFe2++Cu
����2����ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��2Cu2O+Cu2S
| ||
Cu2O Cu2S
Cu2O Cu2S
��3��������Ʒʹ�ø�Ϊ�㷺����������Ʒ�����⣬�������Ҫ�ɷ���
����������
����������
�������ƣ������������ﳣ���Ļ���FeO
FeO
��Fe3O4
Fe3O4
���ѧʽ������4������Ʒ��Ϊ�������γ����ܵ���������Ĥ���������ã����Ǹ�����Ĥ�ױ�����ƻ�������������������������Һ���ã���Ӧ�����ӷ���ʽΪ
Al2O3+2OH-�T2AlO2-+H2O
Al2O3+2OH-�T2AlO2-+H2O
����������1�����ࣨCuSO4��������Ϊͭ������������ͭ��Ӧ�����Ȼ�������ͭ��
��2�����ݷ�Ӧ�� Ԫ�ػ��ϼ۱仯�����жϣ�Ԫ�ػ��ϼ۽��͵���������������
��3������̼�ڵ������Һ�з����绯��ʴ������������������������������Ϊ����������������������
��4������������������������ǿ��ǿ�
��2�����ݷ�Ӧ�� Ԫ�ػ��ϼ۱仯�����жϣ�Ԫ�ػ��ϼ۽��͵���������������
��3������̼�ڵ������Һ�з����绯��ʴ������������������������������Ϊ����������������������
��4������������������������ǿ��ǿ�
����⣺��1�����ࣨCuSO4��������Ϊͭ������������ͭ��Ӧ�����Ȼ�������ͭ��
��Ӧ�����ӷ���ʽΪ��Fe+Cu2+�TFe2++Cu���ʴ�Ϊ��Fe+Cu2+�TFe2++Cu��
��2��2Cu2O+Cu2S
6Cu+SO2������Ӧ��ͭԪ�ػ��ϼ۳�-1�۽��͵�0�ۣ���Ԫ�ػ��ϼ۳�-2�۱仯Ϊ+4�ۣ�������������������Ϊ��Cu2O Cu2S���ʴ�Ϊ��Cu2O Cu2S��
��3������Ʒ�����⣬�������Ҫ�ɷ�������������������������������FeO��Fe3O4��
�ʴ�Ϊ��������������FeO��Fe3O4��
��4���������������������ǿ��ǿ�Ӧ��������������������Һ��������ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪ��Al2O3+2OH-�T2AlO2-+H2O���ʴ�Ϊ��Al2O3+2OH-�T2AlO2-+H2O��
��Ӧ�����ӷ���ʽΪ��Fe+Cu2+�TFe2++Cu���ʴ�Ϊ��Fe+Cu2+�TFe2++Cu��
��2��2Cu2O+Cu2S
| ||
��3������Ʒ�����⣬�������Ҫ�ɷ�������������������������������FeO��Fe3O4��
�ʴ�Ϊ��������������FeO��Fe3O4��
��4���������������������ǿ��ǿ�Ӧ��������������������Һ��������ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪ��Al2O3+2OH-�T2AlO2-+H2O���ʴ�Ϊ��Al2O3+2OH-�T2AlO2-+H2O��
���������⿼��������ͭ�������仯�������ʵ�Ӧ�ã������������������ǽ���ؼ�����Ŀ�ϼ�

��ϰ��ϵ�д�
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
�����Ŀ
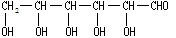 ����дΪGCHO��
����дΪGCHO��