��Ŀ����
��900��Ŀ����кϳɳ�һ�ֺ��硢�ƺ��� (Ħ����2 : 2 : 1) �ĸ�������������̿����� +2��+3��+4 ����ϼ�̬���ڡ�Ϊȷ���ø���������Ļ�ѧʽ���������·�����
�� ȷ��ȡ25.00 mL 0.05301 mol��L��1�IJ�����ˮ��Һ��������ƿ�У�����25 mL����ˮ��5 mL 6 mol��L��1��HNO3��Һ������60~70oC����KMnO4��Һ�ζ�������27.75 mL��д���ζ����̷����ķ�Ӧ�ķ���ʽ������KMnO4��Һ��Ũ�ȡ�
�� ȷ��ȡ0.4460 g������������Ʒ��������ƿ�У���25.00 mL������������Һ��30 mL 6 mol��L��1��HNO3��Һ����60~70oC�³��ҡ����Լ��Сʱ��õ���ɫ����Һ��������KMnO4��Һ�ζ�������10.02 mL������ʵ�������㸴�����������̵ļ�̬�������ø���������Ļ�ѧʽ��д����Ʒ�ܽ���̵ķ�Ӧ����ʽ����֪La��ԭ����Ϊ138.9��
�� ȷ��ȡ25.00 mL 0.05301 mol��L��1�IJ�����ˮ��Һ��������ƿ�У�����25 mL����ˮ��5 mL 6 mol��L��1��HNO3��Һ������60~70oC����KMnO4��Һ�ζ�������27.75 mL��д���ζ����̷����ķ�Ӧ�ķ���ʽ������KMnO4��Һ��Ũ�ȡ�
�� ȷ��ȡ0.4460 g������������Ʒ��������ƿ�У���25.00 mL������������Һ��30 mL 6 mol��L��1��HNO3��Һ����60~70oC�³��ҡ����Լ��Сʱ��õ���ɫ����Һ��������KMnO4��Һ�ζ�������10.02 mL������ʵ�������㸴�����������̵ļ�̬�������ø���������Ļ�ѧʽ��д����Ʒ�ܽ���̵ķ�Ӧ����ʽ����֪La��ԭ����Ϊ138.9��
��2MnO4- + 5C2O42- + 16H+ = 2Mn2+ + 10CO2 + 8H2O
KMnO4��ҺŨ�ȣ� =" 0.01910" ��mol��L-1��
=" 0.01910" ��mol��L-1��
�Ƹ��ݣ��������н�������Ħ����Ϊ La : Ca : Mn =" 2" : 2 : 1����Ƶ�����̬�ֱ�Ϊ+3��+2���̵�����̬Ϊ +2 ~ +4�������ж�
����������Ļ�ѧʽΪLa2Ca2MnO6+x, ����x = 0~1��
������
����C2O42��������25.00 mL ��0.05301 mol��L��1=" 1.3253" mmol
��Ʒ�ܽ�ζ����ĸ�����أ�10.02 mL ��0.01910 mol��L��1 =" 0.1914" mmol
��Ʒ�ܽ��ʣ��C2O42����: 0.1914 mmol �� =" 0.4785" mmol
=" 0.4785" mmol
��Ʒ�ܽ���������ĵ�C2O42����: 1.3253 mmol ��0.4785 mmol =" 0.8468" mmol
�����������У�C2O42����ΪCO2�������ӣ�
2 �� 0.8468 mmol =" 1.694" mmol
���������x�ķ�����
��1�����̷���
����������(La2Ca2MnO6+x)��Ʒ�����ʵ���Ϊ��
0.4460 g / [(508.9 + 16.0 x) g��mol-1]
La2Ca2MnO6+x�У��̵ļ�̬Ϊ: [2 ��(6+x) -2 ��3 -2��2] = (2+2x)
�����������̵ļ�̬�仯Ϊ�� (2+2 x- 2) =" 2" x
�̵õ�������C2O42������������ȣ�
2 x�� 0.4460 g / [(508.9 + 16.0 x) g��mol-1] =" 2" ��0.8468 ´ 10-3 mol
x =" 1.012" ��1
��2�����Է�
��Ϊ���������������൱����C2O42-���ɼ��̵ļ�̬�϶�������+2�ۡ������̵ļ�̬Ϊ+3�ۣ���Ӧ������Ļ�ѧʽΪLa2Ca2MnO6.5, �˻�ѧʽʽ��Ϊ516.9 g��mol-1, ��ȡ��Ʒ�����ʵ���Ϊ��
0.4460 g / (516.9 g��mol-1) =" 8.628" ��10-4 mol
�������������̵ļ�̬�仯Ϊ
1.689�� 10-3 mol / (8. 628��10-4 mol) =" 1.96"
���ڸ����������еļ�̬Ϊ�� 2 + 1.96 =" 3.96 "
3.96��3���ܴ�+3�ۼ��費������
�������ʾMn���ӽ���+4�ۡ�
����MnΪ+4��, ��Ӧ������Ļ�ѧʽΪLa2Ca2MnO7, �˻�ѧʽʽ��Ϊ524.9 g��mol-1��
���ڸ����������еļ�̬Ϊ��2 + 2 �� 0.8468 ��10-3 / (0.4460 / 524.9) =" 3.99" �� 4
���������Ǻϣ��ɼ��ڸ����������У�MnΪ+4�ۡ�
�ø���������Ļ�ѧʽΪLa2Ca2MnO7
�������̵ķ�Ӧ����ʽΪ��
La2Ca2MnO7 + C2O42- + 14H+ = 2La3+ + 2Ca2+ + Mn2+ + 2CO2 + 7H2O
KMnO4��ҺŨ�ȣ�
 =" 0.01910" ��mol��L-1��
=" 0.01910" ��mol��L-1���Ƹ��ݣ��������н�������Ħ����Ϊ La : Ca : Mn =" 2" : 2 : 1����Ƶ�����̬�ֱ�Ϊ+3��+2���̵�����̬Ϊ +2 ~ +4�������ж�
����������Ļ�ѧʽΪLa2Ca2MnO6+x, ����x = 0~1��
������
����C2O42��������25.00 mL ��0.05301 mol��L��1=" 1.3253" mmol
��Ʒ�ܽ�ζ����ĸ�����أ�10.02 mL ��0.01910 mol��L��1 =" 0.1914" mmol
��Ʒ�ܽ��ʣ��C2O42����: 0.1914 mmol ��
 =" 0.4785" mmol
=" 0.4785" mmol��Ʒ�ܽ���������ĵ�C2O42����: 1.3253 mmol ��0.4785 mmol =" 0.8468" mmol
�����������У�C2O42����ΪCO2�������ӣ�
2 �� 0.8468 mmol =" 1.694" mmol
���������x�ķ�����
��1�����̷���
����������(La2Ca2MnO6+x)��Ʒ�����ʵ���Ϊ��
0.4460 g / [(508.9 + 16.0 x) g��mol-1]
La2Ca2MnO6+x�У��̵ļ�̬Ϊ: [2 ��(6+x) -2 ��3 -2��2] = (2+2x)
�����������̵ļ�̬�仯Ϊ�� (2+2 x- 2) =" 2" x
�̵õ�������C2O42������������ȣ�
2 x�� 0.4460 g / [(508.9 + 16.0 x) g��mol-1] =" 2" ��0.8468 ´ 10-3 mol
x =" 1.012" ��1
��2�����Է�
��Ϊ���������������൱����C2O42-���ɼ��̵ļ�̬�϶�������+2�ۡ������̵ļ�̬Ϊ+3�ۣ���Ӧ������Ļ�ѧʽΪLa2Ca2MnO6.5, �˻�ѧʽʽ��Ϊ516.9 g��mol-1, ��ȡ��Ʒ�����ʵ���Ϊ��
0.4460 g / (516.9 g��mol-1) =" 8.628" ��10-4 mol
�������������̵ļ�̬�仯Ϊ
1.689�� 10-3 mol / (8. 628��10-4 mol) =" 1.96"
���ڸ����������еļ�̬Ϊ�� 2 + 1.96 =" 3.96 "
3.96��3���ܴ�+3�ۼ��費������
�������ʾMn���ӽ���+4�ۡ�
����MnΪ+4��, ��Ӧ������Ļ�ѧʽΪLa2Ca2MnO7, �˻�ѧʽʽ��Ϊ524.9 g��mol-1��
���ڸ����������еļ�̬Ϊ��2 + 2 �� 0.8468 ��10-3 / (0.4460 / 524.9) =" 3.99" �� 4
���������Ǻϣ��ɼ��ڸ����������У�MnΪ+4�ۡ�
�ø���������Ļ�ѧʽΪLa2Ca2MnO7
�������̵ķ�Ӧ����ʽΪ��
La2Ca2MnO7 + C2O42- + 14H+ = 2La3+ + 2Ca2+ + Mn2+ + 2CO2 + 7H2O
�Ŵ˹���Ϊ����Һ�ı궨�����ݹ�ϵʽ5C2O42����2MnO4������C2O42-����Һ�궨��MnO4-��Ũ�ȡ�
�Ƹ÷��������������������ķ��ζ����ͣ���KMnO4�ζ�������C2O42-�����ݵ��ӵ�ʧ�غ��ϵ�����г���ʽ
MnԪ�ص�ʧ��������5��n(MnO4-)=2��n(C2O42-)
���MnԪ�ص�ʧ���ӵ����ʵ���Ϊ1.694 mmol
�踴���������ͨʽ��La2Ca2MnO6+x���������������Mn�����ʵ�����������õζ���������Ԫ���������ı仯������ȷ�����������̵���������
�Ƹ÷��������������������ķ��ζ����ͣ���KMnO4�ζ�������C2O42-�����ݵ��ӵ�ʧ�غ��ϵ�����г���ʽ
MnԪ�ص�ʧ��������5��n(MnO4-)=2��n(C2O42-)
���MnԪ�ص�ʧ���ӵ����ʵ���Ϊ1.694 mmol
�踴���������ͨʽ��La2Ca2MnO6+x���������������Mn�����ʵ�����������õζ���������Ԫ���������ı仯������ȷ�����������̵���������

��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д�
�����Ŀ

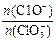 2���>������<����=������
2���>������<����=������ ����n(C1��)= mol���ú�a�Ĵ���ʽ����ʾ����
����n(C1��)= mol���ú�a�Ĵ���ʽ����ʾ���� 3Fe��4CO2�У�
3Fe��4CO2�� 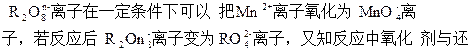
 MnO2+K2O+O2(240 ��)������˵������ȷ����( )
MnO2+K2O+O2(240 ��)������˵������ȷ����( )