��Ŀ����
����Ŀ����������������Ҫ���л��ϳ��м��壬��һ�ֺϳ�·�����£�
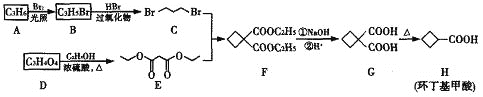
��ش��������⣺
(1)����������ķ���ʽΪ____________________��
(2)������������ԭ��A��D��һϵ�з�Ӧ�Ƶã�AΪϩ������A������Ϊ______��D���ʵĹ�����Ϊ_______��
(3)д��D��E�Ļ�ѧ����ʽ________________________��
(4)C+E��F�ķ�Ӧ����Ϊ_________________________��
(5)������WΪH��ͬ���칹�壬�ܷ���������Ӧ��ֻ������һ�ֹ����ţ������з���������W�Ľṹ��ʽΪ_____________��
(6)���������ϳ�·�ߣ��� ![]() ��EΪԭ��(���Լ���ѡ)������Ʊ�
��EΪԭ��(���Լ���ѡ)������Ʊ�![]() �ĺϳ�·�ߣ�__________��
�ĺϳ�·�ߣ�__________��
���𰸡�C5H8O2 ��ϩ �Ȼ� HOOC-CH2-COOH+2CH3CH2OH![]() C2H5OOCCH2COOC2H5+2H2O ȡ����Ӧ
C2H5OOCCH2COOC2H5+2H2O ȡ����Ӧ ![]()
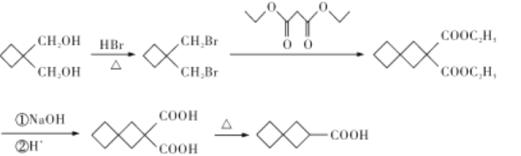
��������
����ϳɷ���������C�Ľṹ���Ƴ�BΪCH2=CH-CH2Br��AΪCH2=CH-CH3��D���Ҵ�����������Ӧ����E����E�Ľṹ��ʽ���Ƴ�DΪ�����ᣬ�ṹ��ʽΪHOOC-CH2-COOH��
����ϳɷ���������C�Ľṹ���Ƴ�BΪCH2=CH-CH2Br��AΪCH2=CH-CH3��D���Ҵ�����������Ӧ����E����E�Ľṹ��ʽ���Ƴ�DΪ�����ᣬ�ṹ��ʽΪHOOC-CH2-COOH��
��1�������л�����̼ԭ�ӳɼ��ص㣬����������ķ���ʽΪC5H8O2��
��2����������������A�Ľṹ��ʽΪCH2=CH��CH3����ѧ����Ϊ��ϩ��D�Ľṹ��ʽΪHOOCCH2COOH��������Ϊ�Ȼ���
��3��D����E����������Ӧ���仯ѧ��Ӧ����ʽΪHOOC-CH2-COOH+2CH3CH2OH![]() C2H5OOCCH2COOC2H5+2H2O��
C2H5OOCCH2COOC2H5+2H2O��
��4���Ա�C��E��F�Ľṹ��ʽ��C��E��F��Ӧ����ʽΪ![]() +
+![]() ��
��![]() +2HBr���䷴Ӧ����Ϊȡ����Ӧ��
+2HBr���䷴Ӧ����Ϊȡ����Ӧ��
��5����������һ�ֹ����ţ����ܷ���������Ӧ��˵����д��ͬ���칹���к���HCOO�����ţ�������������![]() ��
��
��6����![]() �ͻ�����E���������������Ϊԭ�ϣ��Ʊ�
�ͻ�����E���������������Ϊԭ�ϣ��Ʊ�![]() ��������ϳɷ��������Ʊ�
��������ϳɷ��������Ʊ�![]() ����Ҫ
����Ҫ![]() ��
��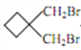 ��
��![]() �����������������Ӧ����
�����������������Ӧ����![]() ��
��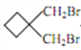 ����
����![]() ��HBrȡ�����ɣ��ʺϳ�·��Ϊ��
��HBrȡ�����ɣ��ʺϳ�·��Ϊ�� ��
��








