��Ŀ����
�������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ��C3H6����
��1����������ɵñ�ϩ��
��֪��C3H8��g����CH4��g��+HC��CH��g��+H2��g����H1=156.6kJ?mol-1
CH3CH=CH2��g����CH4��g��+HC��CH��g����H2=32.4kJ?mol-1
����ͬ�����£���ӦC3H8��g����CH3CH=CH2��g��+H2��g���ġ�H=
��2��̼�⻯������ȫȼ������CO2��H2O�����³�ѹ�£������е�CO2����ˮ���ﵽƽ��ʱ����Һ��pH=5.60��c��H2CO3��=1.5��10-5 mol?L-1��������ˮ�ĵ��뼰H2CO3�ĵڶ������룬��H2CO3?HCO3-+H+�ĵ���ƽ�ⳣ��Ka1=
��3����1LŨ��Ϊc mol/LCH3COOH��Һ�У�CH3COOH���ӡ�H+��CH3COO-�������ʵ���֮��Ϊnc mol����CH3COOH�ڸ��¶��µĵ����Ϊ
��1����������ɵñ�ϩ��
��֪��C3H8��g����CH4��g��+HC��CH��g��+H2��g����H1=156.6kJ?mol-1
CH3CH=CH2��g����CH4��g��+HC��CH��g����H2=32.4kJ?mol-1
����ͬ�����£���ӦC3H8��g����CH3CH=CH2��g��+H2��g���ġ�H=
124.2
124.2
kJ?mol-1����2��̼�⻯������ȫȼ������CO2��H2O�����³�ѹ�£������е�CO2����ˮ���ﵽƽ��ʱ����Һ��pH=5.60��c��H2CO3��=1.5��10-5 mol?L-1��������ˮ�ĵ��뼰H2CO3�ĵڶ������룬��H2CO3?HCO3-+H+�ĵ���ƽ�ⳣ��Ka1=
4.2��10-7
4.2��10-7
������֪��10-5.60=2.5��10-6����3����1LŨ��Ϊc mol/LCH3COOH��Һ�У�CH3COOH���ӡ�H+��CH3COO-�������ʵ���֮��Ϊnc mol����CH3COOH�ڸ��¶��µĵ����Ϊ
n-1
n-1
��100%��������1�������Ȼ�ѧ����ʽ��˹���ɼ���õ���
��2���������ƽ�ⳣ������ƽ��״̬����ƽ��Ũ���ݴη��˻����Ե��������ƽ��Ũ�ȴﵽ��
��3����������ѵ���Ĵ���ռ��ʼ����İٷֱȣ�
��2���������ƽ�ⳣ������ƽ��״̬����ƽ��Ũ���ݴη��˻����Ե��������ƽ��Ũ�ȴﵽ��
��3����������ѵ���Ĵ���ռ��ʼ����İٷֱȣ�
����⣺��1����C3H8��g����CH4��g��+HC��CH��g��+H2��g����H1=156.6kJ?mol-1
��CH3CH=CH2��g����CH4��g��+HC��CH��g����H2=32.4kJ?mol-1
���ݸ�˹���ɢ�-�ڵõ�C3H8��g����CH3CH=CH2��g��+H2��g����H=+124.2KJ/mol
����ͬ�����£���ӦC3H8��g����CH3CH=CH2��g��+H2��g����H=+124.2KJ/mol��
�ʴ�Ϊ��+124.2��
��2��̼�⻯������ȫȼ������CO2��H2O�����³�ѹ�£������е�CO2����ˮ���ﵽƽ��ʱ����Һ��pH=5.60��c��H+��=c��HCO3-��=10-5.6mol/L��c��H2CO3��=1.5��10-5 mol?L-1��������ˮ�ĵ��뼰H2CO3�ĵڶ������룬��H2CO3?HCO3-+H+�ĵ���ƽ�ⳣ��Ka1=
=
=4.2��10-7��
�ʴ�Ϊ��4.2��10-7��
��3����1LŨ��Ϊc mol/LCH3COOH��Һ�У�CH3COOH���ӡ�H+��CH3COO-�������ʵ���֮��Ϊnc mol�������Ĵ������ʵ���ΪX
CH3COOH?CH3COO-+H+
��ʼ����mol�� c 0 0
�仯����mol�� X X X
ƽ������mol�� c-X X X
c-X+X+X=nc
X=c��n-1��
��CH3COOH�ڸ��¶��µĵ����=
��100%=��n-1����100%
�ʴ�Ϊ��n-1
��CH3CH=CH2��g����CH4��g��+HC��CH��g����H2=32.4kJ?mol-1
���ݸ�˹���ɢ�-�ڵõ�C3H8��g����CH3CH=CH2��g��+H2��g����H=+124.2KJ/mol
����ͬ�����£���ӦC3H8��g����CH3CH=CH2��g��+H2��g����H=+124.2KJ/mol��
�ʴ�Ϊ��+124.2��
��2��̼�⻯������ȫȼ������CO2��H2O�����³�ѹ�£������е�CO2����ˮ���ﵽƽ��ʱ����Һ��pH=5.60��c��H+��=c��HCO3-��=10-5.6mol/L��c��H2CO3��=1.5��10-5 mol?L-1��������ˮ�ĵ��뼰H2CO3�ĵڶ������룬��H2CO3?HCO3-+H+�ĵ���ƽ�ⳣ��Ka1=
| c(H+)c(HCO3-) |
| c(H2CO3) |
| 10-5.6��10-5.6 |
| 1.5��10-5 |
| 2.5��10-6��2.5��10-6 |
| 1.5��10-5 |
�ʴ�Ϊ��4.2��10-7��
��3����1LŨ��Ϊc mol/LCH3COOH��Һ�У�CH3COOH���ӡ�H+��CH3COO-�������ʵ���֮��Ϊnc mol�������Ĵ������ʵ���ΪX
CH3COOH?CH3COO-+H+
��ʼ����mol�� c 0 0
�仯����mol�� X X X
ƽ������mol�� c-X X X
c-X+X+X=nc
X=c��n-1��
��CH3COOH�ڸ��¶��µĵ����=
| c(n-1) |
| c |
�ʴ�Ϊ��n-1
���������⿼�����Ȼ�ѧ����ʽ��˹���ɼ���Ӧ�ã��������ƽ�ⳣ������͵���ȼ���Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 HCO3-+H+��ƽ�ⳣ��K1=
HCO3-+H+��ƽ�ⳣ��K1= CO32-+H+��HCO3-+H2O
CO32-+H+��HCO3-+H2O H2CO3+OH-��
H2CO3+OH-��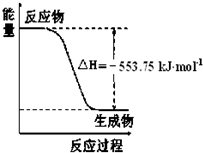 ��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵���
��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵���