��Ŀ����
����Ŀ���������ʵ�����A��B�����2L���ܱ������У��������з�Ӧ��3A(g)��B(g) ![]() xC(g)��2D(g)����2min����D��Ũ��Ϊ0.5mol��L��1��c(A)�Uc(B)��3�U5�� ��C��ʾ��ƽ������v(C)��0.25mol��L��1��min��1������˵������ȷ����( )
xC(g)��2D(g)����2min����D��Ũ��Ϊ0.5mol��L��1��c(A)�Uc(B)��3�U5�� ��C��ʾ��ƽ������v(C)��0.25mol��L��1��min��1������˵������ȷ����( )
A. ��Ӧ����v(B)��0.125mol��L��1��min��1
B. �÷�Ӧ����ʽ�У�x��2
C. 2minʱ��C�����ʵ���Ϊ1.0mol
D. 2minʱ��A��Ũ��Ϊ1.5mol��L��1
���𰸡�D
��������
A.V(D)=![]() =0.25mol/(L��min)�����ݷ���ʽ��֪V(B):V(D)=1:2������V(D)=0.25 mol/(L��min)������V(B)=0.125 mol/(L��min)��A��ȷ��
=0.25mol/(L��min)�����ݷ���ʽ��֪V(B):V(D)=1:2������V(D)=0.25 mol/(L��min)������V(B)=0.125 mol/(L��min)��A��ȷ��
B.����V(D)=0.25 mol/(L��min)��v(C)= 0.25 mol/(L��min)�����ʱȵ��ڻ�ѧ����ʽ�����ʵĻ�ѧ�������ıȣ����ݷ���ʽ��֪V(C):V(D)=x��2=1:1������x=2��B��ȷ��
C.����V(C)=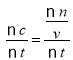 =0.25 mol/(L��min)������2minʱ��C�����ʵ���n=0.25 mol/(L��min)��2min��2L=1.0mol��C��ȷ��
=0.25 mol/(L��min)������2minʱ��C�����ʵ���n=0.25 mol/(L��min)��2min��2L=1.0mol��C��ȷ��
D.���跴Ӧ��ʼʱA��B�����ʵ���Ϊamol����Ӧ������D���ʵ���Ϊn(D)=0.5mol/L��2L=1.0mol
���淴Ӧ 3A(g) ��B(g) ![]() xC(g)��2D(g)��
xC(g)��2D(g)��
n(��ʼ)mol a a 0 0
n(�仯)mol 1.5 0.5 1.0 1.0
n(2��)mol a-1.5 a-0.5 1.0 1.0
������ͬһ�����ڽ��У����ʵ����ıȵ���Ũ�ȱȣ�����(a-1.5)��(a-0.5)= 3�U5�����a=3mol������2minʱA��Ũ��Ϊ(3mol-1.5mol)��2min=0.75 mol/(L��min)��D����
�ʺ���ѡ����C��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�