��Ŀ����
(8��)�������������������ǿ�������������ɷ�����H1N1���С�
(1)��̼������һ���ж�����;��������ϵ��̬Ư������ѧʽ�ɱ�ʾΪNa2CO3��3H2O2��������Na2CO3��H2O2��˫�����ʡ�
��H2O2��ʱ����Ϊ��ҵ��Һ���������������ɿ�ҵ��Һ�е��軯��(��NaCN)�������·�Ӧʵ�֣�NaCN��H2O2��H2O===A��NH3������������A�Ļ�ѧʽΪ_______________��
��ijǿ���Է�Ӧ��ϵ�У���Ӧ��������ﹲ�������ʣ�O2��MnO��H2O��Mn2����H2O2��H������֪�÷�Ӧ��H2O2ֻ���������¹��̣�H2O2�� O2��д���÷�Ӧ�����ӷ���ʽ��_________��
(2)ij��Ȼ��Ļ�ѧʽ�ɱ�ʾΪ:aNa2CO3��bNaHCO3��2H2O��ȡm g��Ȼ������ˮ�����Һ��������Һ����μ���1 mol/L�����ᣬ��״���²�����CO2������������������֮��Ĺ�ϵijͬѧ����������ͼ��ʾ��A��B���ߣ��Իش��������⣺
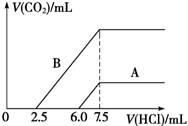
��_______������ȷ����Ȼ��Ļ�ѧʽΪ___________��
�ۼ���������CO2�������(��״��)�����ֵΪ_________________mL��
(3)Ư����������(NaClO2)�ڳ�����ڰ����ɱ���һ�ꡣ
������ȶ��ɷֽ⣬��Ӧ�����ӷ���ʽΪ��HClO2�D��ClO2����H����Cl����H2O(δ��ƽ)���ڸ÷�Ӧ�У�����1 mol ClO2����ʱת�Ƶĵ��Ӹ�����________��
(1)��NaHCO3 (1��) ��2MnO��5H2O2��6H��===2Mn2����8H2O��5O2��(2��)
(2)B; (1��)�� Na2CO3��NaHCO3��2H2O��(1��) 112(1��) (3)6.02��1023(1��)
����������1���ٸ���ԭ���غ��֪AӦ����̼�����ơ�
��H2O2�� O2��˵��˫��ˮ�ǻ�ԭ��������MnO4��������������ʽΪ2MnO��5H2O2��6H��===2Mn2����8H2O��5O2����
��2������̼���ƺ����ᷴӦ�Ƿֲ����еģ���Na2CO3��HCl=NaHCO3��NaCl��NaHCO3��HCl=NaCl��H2O��CO2��������ں���̼�����Ƶ������£�����CO2ʱ���ĵ�����࣬��B������ȷ������ͼ���֪����̼���Ʒ�Ӧ������ʱ2.5ml�����Ժ�ԭ̼�����Ʒ�Ӧ��������7.5ml��2.5ml��2.5ml��2.5ml�����a��b�ı�ֵ��1�U1�ģ���ѧʽΪNa2CO3��NaHCO3��2H2O������ͼ���֪����CO2ʱ���ĵ�������5ml����������CO2��0.005mol����״���µ������0.112L��
��3��HClO2����Ԫ�صĻ��ϼ��ǣ�3�ۣ���Ӧ�����ߵ���4�ۣ����Ե���1 mol ClO2����ʱת�Ƶĵ�����1mol��������6.02��1023��

 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
 ��ط�Ӧ�����ӷ���ʽΪ ��
��ط�Ӧ�����ӷ���ʽΪ �� ���ڵ���ij������Ӿ���ļ���ˮ�������ʱ��
���ڵ���ij������Ӿ���ļ���ˮ�������ʱ��