��Ŀ����
����о�������п���Ŵ�ѧ��������Ҫ���ã�ȱп����������ٶۣ�������˥�ˣ����пҲ��Ϊ������Ԫ�ء�����һ����п����Ļ�����ڸ��������������¼��ȣ� �ڷ�Ӧ�����ȴ���м���������ϡ�������2.24L���壨����£�����˵������ȷ����
| A��������������С��9.7��ʱ������ijɷ�ΪH2S��H2 |
| B��ԭ������е�ZnΪ6.5�� |
| C������������������9.7��ʱ������ijɷֽ�ΪH2S |
| D����ȼ�����ʵ���H2S��O2�Ļ�����壬���ɵĺ�����Ϊ������CS2�ĵ��� |
D
Zn��S��Ӧ����ZnS���䷽��ʽΪ��Zn+S==ZnS����������ϡ���ᷴӦ�ķ���ʽΪ��ZnS + H2SO4 =====ZnSO4 + H2S��Zn��ϡ���ᷴӦ�ķ���ʽΪ��Zn + H2SO4===== ZnSO4 + H2������Zn�Ƿ������ϡ���ᷴӦ������������Ĺ�ϵ��Ϊ1��1�������壻2.24L���壨����£������ʵ���Ϊ0.1mol�����������ʵ���Ϊ0.1mol��
������ֻ��ZnS����ԭ����������Ϊ9.7g����������ΪH2S��
��������ZnS������Zn����������������С��9.7�ˣ�����ijɷ�ΪH2S��H2��
��������ZnS������S���������������ش���9.7�ˣ�����ijɷ�ΪH2S
����A��ȷ��B��ȷ����0.1mol�������ɣ�ԭ������е�Zn��Ϊ6.5�ˣ�
C��ȷ������������������9.7��ʱ������ijɷֽ�ΪH2S��D����H2S��O2��Ӧ�ķ���ʽ�У�2H2S + O2===== S + 2H2O��2H2S + 3O2===== 2SO2 + 2H2O������ȼ�����ʵ���H2S��O2�Ļ�����壬���ɵĺ�����ΪSO2��S��
������ֻ��ZnS����ԭ����������Ϊ9.7g����������ΪH2S��
��������ZnS������Zn����������������С��9.7�ˣ�����ijɷ�ΪH2S��H2��
��������ZnS������S���������������ش���9.7�ˣ�����ijɷ�ΪH2S
����A��ȷ��B��ȷ����0.1mol�������ɣ�ԭ������е�Zn��Ϊ6.5�ˣ�
C��ȷ������������������9.7��ʱ������ijɷֽ�ΪH2S��D����H2S��O2��Ӧ�ķ���ʽ�У�2H2S + O2===== S + 2H2O��2H2S + 3O2===== 2SO2 + 2H2O������ȼ�����ʵ���H2S��O2�Ļ�����壬���ɵĺ�����ΪSO2��S��

��ϰ��ϵ�д�
�����Ŀ
 ���ᷴӦ)��
���ᷴӦ)��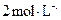 ���ᡢ5%NaOH��Һ������
���ᡢ5%NaOH��Һ������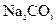 ��Һ������
��Һ������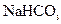 ��Һ������ˮ��
��Һ������ˮ��
 ����ͬһˮƽ���ϣ�������ʱ�ҹܵ�Һ�������ܵ�Һ�棬Ӧ���еIJ�����
����ͬһˮƽ���ϣ�������ʱ�ҹܵ�Һ�������ܵ�Һ�棬Ӧ���еIJ�����  ��
��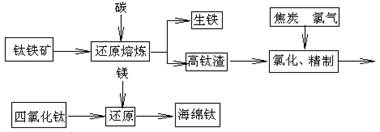
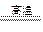 TiO2+Fe+CO��,��FeTiO3�������������У��ѵĻ��ϼ�Ϊ ��
TiO2+Fe+CO��,��FeTiO3�������������У��ѵĻ��ϼ�Ϊ ��