��Ŀ����
�������ӷ���ʽ������ȷ����(����)
A�������������������:Fe(OH)3+3H+ Fe3++3H2O Fe3++3H2O |
B��С�մ���Һ�ʼ��Ե�ԭ��:HC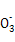 +H2O +H2O H3O++C H3O++C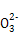 |
C��ϡ�����м�����������м:3Fe+8H++2N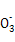  3Fe2++2NO��+4H2O 3Fe2++2NO��+4H2O |
D�����������[NH4Al(SO4)2]��Һ�еμ�����Ba(OH)2��Һ:N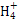 +Al3++2S +Al3++2S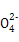 +2Ba2++5OH- +2Ba2++5OH- Al Al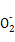 +2BaSO4��+NH3��H2O+2H2O +2BaSO4��+NH3��H2O+2H2O |
C
����

�������ӷ���ʽ��д��ȷ����
| A������ͨ�������Һ�У� CH3COOH+NH3��CH3COONH4 |
| B������ϡ���ᷴӦ�� 2Fe+6H+ = 2Fe3++3H2�� |
| C������������ϡ��� Ba2++2OH-+2H++SO42-=BaSO4��+2H2O |
| D�����Ƽ���ˮ�У� Na��2H2O=Na++2OH-��H2�� |
�ձ���ʢ��100 mL 1 mol/L��NaHSO4��Һ,��������εμ�1 mol/L��Ba(OH)2��Һ,�ձ���ijЩ����(����)�����ʵ����ı仯������ͼ������˵������ȷ����(����)
| A������a��ʾBa2+�����ʵ����ı仯 |
| B������c��ʾOH-�����ʵ����ı仯 |
C������Ba(OH)2��Һ50 mL��Ӧ�����ӷ���ʽΪBa2++OH-+H++SO42�� BaSO4��+H2O BaSO4��+H2O |
D������Ba(OH)2��Һ����50 mL��,��Ӧ�����ӷ���ʽΪOH-+H+ H2O H2O |
���н�����������ʵ������ӷ���ʽ����ȷ����(����)
A��NaClO��Һ�ʼ��ԣ�ClO����H2O HClO��OH�� HClO��OH�� |
B�����ˮ�еμӱ���FeCl3��Һ�ƽ��壺Fe3����3H2O Fe(OH)3(����)��3H�� Fe(OH)3(����)��3H�� |
| C�������ᴿ�г�ȥMg2����Mg2����2OH��=Mg(OH)2�� |
| D����ҵ�Ͼ�����ͭ��������Ӧ��Cu��2e��=Cu2�� |
��֪����ƽ�ⳣ����H2CO3��HClO��HCO3���������ԣ�HClO��Cl2��Br2��Fe3����I2�������й����ӷ�Ӧ�����ӷ���ʽ�������У���ȷ����(����)
| A����FeI2��Һ�еμ�������ˮ����Ӧ�����ӷ���ʽΪ2Fe2����Cl2=2Fe3����2Cl�� |
| B������ˮ�м��������Ȼ�������Һ��ʹ��ˮ�����ɫ |
| C����NaClO��Һ��ͨ������CO2�����ӷ���ʽ��2ClO����CO2��H2O=2HClO��CO32�� |
| D����ʹpH��ֽ�����ɫ����Һ�У�Fe3����Cl����Ba2����Br���ܴ������� |
��������[Al2Fe(SO4)4��xH2O]����ˮ��,�õ�dz��ɫ��Һ,�йظ���Һ��������ȷ����(�� ��)
| A���������о�ˮ����,��ˮ��Һ������ |
B������Һ��:2c(Al3+)+c(Fe2+)+c(H+)=4c(S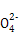 )+c(OH-) )+c(OH-) |
| C���ڿ�����,����Һ���ɲ�����,������õIJ�������ΪAl2O3��Fe2O3 |
D�������Һ�м���Ba(OH)2��Һ,��S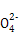 ��ȫ����ʱ,Al3+��Fe2+ǡ�ñ���ȫ���� ��ȫ����ʱ,Al3+��Fe2+ǡ�ñ���ȫ���� |
�������ӷ���ʽ��д��ȷ���ǣ�������
| A����ͭм����Fe3����Һ�У�Fe3����Cu=Fe2����Cu2�� |
B��NH4HCO3��Һ�����KOHŨ��Һ���ȣ�NH4+��OH�� NH3����H2O NH3����H2O |
| C��ϡ�����������м��Ӧ�� Fe��4H����NO3-=Fe3����NO����2H2O |
| D��KI��Һ��H2SO4�ữ��H2O2��Һ��ϣ�2I����H2O2��2H��=I2��2H2O |
���н���ʵ����ʵ�ķ�Ӧ����ʽ����ȷ����(����)
| A��ʢ���ռ���Լ�ƿ�����ò�������SiO2��2NaOH=Na2SiO3��H2O |
| B�����ռ���Һ����������Cl2��2OH��=Cl����ClO����H2O |
| C����KSCN��Һ����Fe3����Fe3����3SCN��=Fe(SCN)3 |
| D������KI������Һ���ú������4I����O2��2H2O=2I2��4OH�� |
ij��Һ���ܺ������������еļ���(��������Һ������H����OH��)��Na����NH4+��SO42����CO32����NO3����ȡ200 mL����Һ��������ֳ����ݣ��ֱ�������ʵ�顣
ʵ��һ����һ�ݼ��������ռ���ȣ������������ڱ�״����Ϊ224 mL��
ʵ������ڶ����ȼ������������ᣬ�������ټ�������BaCl2��Һ���ó���2.33 g��
����˵����ȷ����(����)
| A������Һ���ܺ���Na�� |
| B������Һһ������NH4+��SO42����CO32����NO3�� |
| C������Һһ��������NO3�� |
| D������Һһ������Na������c(Na��)��0.1 mol��L��1 |