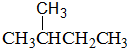��Ŀ����
(11��)���ʵķ�����ж��֡������ͬһ�����ʿ������ڲ�ͬ�����з��࣬Ҳ���ù��ɡ��Ƚϵȷ��������ʽ��з���ȣ����������Ŀ��
(1)���Т��������ڿ������ۼ�ʽ̼��ͭ��������ء�����ˮ��������þ�����Ȼ��ơ�����ˮ���ⴿ����(CH3COOH)��?���ᱵ��?�Ҵ���?ͭ�����ʡ�
�������ڵ��ʵ���________�����ڻ��������________������________���ں��������________������������ڻ�������________�����ڵ���ʵ���________�����ڷǵ���ʵ���________(�������)��
(2)���������������ʣ�ÿ���ж���һ�����ʸ������������ڲ�ͬ�����ࡣ���������ʺͷ�������(ѡ�����������������ʲ�֮ͬ��)д��������Ӧ�ı����ڡ��������ʷֱ�Ϊ��
��O2��Cl2��S��N2
��Fe��Na��Al��Si
| ��� | ��ѡ�������� | �������� |
| �ڢ��� | | |
| �ڢ��� | | |
(1)�٢�?���ۢܢޢߢ��??���ۢܢޢߢ�??���ޢߡ��ڢᡡ�ۢܢޢߢ��?��?
(2)��S�������µ�״̬
��Si����Ϊ�ǽ���?
����

 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�(11��)�±������������г��������ʣ������г������ǵģ���Ҫ���ɷ֡�
|
��� |
�� |
�� |
�� |
�� |
�� |
�� |
�� |
|
���� |
�ƾ� |
���� |
��� |
ʳ�� |
ͭ���� |
�������� |
�մ� |
|
��Ҫ �ɷ� |
CH3CH2OH |
CH3COOH |
NaOH |
NaCl |
Cu |
SO2 |
Na2CO3 |
��1������Ա��Т١��ߵ���Ҫ�ɷֽ��з��ࣨ���ţ���
�����ε��� �����ڵ���ʵ��� �����ڷǵ���ʵ��� ��
��2��д�������ڵ�ˮ��Һ��߷�Ӧ�Ļ�ѧ����ʽ ��
����������߷�Ӧ�����ӷ���ʽ ��
��ijͬѧ�âݺ�Ũ���Ṳ�����Ʊ��ޣ���ѧ����ʽΪ��
Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
��������ת�Ƶķ������Ŀ��������ת��0.1molʱ�����Ļ�ԭ��������Ϊ g��
 ��
��