��Ŀ����
��ⷨ�ٽ����ʯ����Ҫ�ɷ���Mg2SiO4���̶�CO2�IJ��ֹ���������ͼ1��
��֪��Mg2SiO4��s��+4HCl��aq���T2MgCl2��aq��+SiO2 ��s��+2H2O��l����H=-49.04kJ?mol-1
��1��ij���ʯ�������Mg9FeSi5O20�������������ʽ�ɱ�ʾΪ
��2����̼ʱ��Ҫ��Ӧ�ķ���ʽΪNaOH��aq��+CO2 ��g��=NaHCO3 ��aq�����÷�Ӧ���Է����е�ԭ����
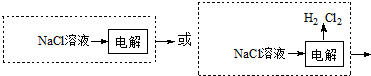
��3������ͼ1���ڲ���һ����ҵ��������
��4������������Ҳ����������̼������
a��CaCl2 b��H2NCH2COONa c����NH4��2CO3
��5����ͼ2֪��90�������A�ܽ�Ч���½���������ԭ��
��6�������������ü�ʽ̼��þ��Ʒ�к�������NaCl��Fe2O3��Ϊ�ᴿ���ɲ�ȡ�Ĵ�ʩ����Ϊ�����ܽ��������Һ���г����������Բ�Ʒ����ϴ�Ӵ������жϲ�Ʒϴ���IJ�����
��������1���������ǹ��ɵؿ���ʯ����Ҫ�ɷ֣���ѧ�ϳ��ö�����������������ʽ��ʾ����ɣ����磺þ���ʯ��Mg2SiO4������2MgO?SiO2��ʾ���ɿ�����д��Ҫ��֤ԭ�����������ϼ۲��䣬�����ϼ۷ֱ�д��������Ļ�ѧʽ�����ж��ԭ�ӣ���ǰ�����ϵ����ʹ֮��Ϊ�������ݴ˽��з������
��2����������ȵġ������ӵĹ��������ڷ�Ӧ���Է����У�
��3����ҵ�����ȵ�ⱥ��ʳ��ˮ�����ռ
��4�������ܺͶ�����̼֮�䷴Ӧ�����������̶�������̼���ش�
��5���¶ȶԻ�ѧ��Ӧƽ���ƶ���Ӱ��֪ʶ���ش�
��6�������ӵļ����������ữ����������
��2����������ȵġ������ӵĹ��������ڷ�Ӧ���Է����У�
��3����ҵ�����ȵ�ⱥ��ʳ��ˮ�����ռ
��4�������ܺͶ�����̼֮�䷴Ӧ�����������̶�������̼���ش�
��5���¶ȶԻ�ѧ��Ӧƽ���ƶ���Ӱ��֪ʶ���ش�
��6�������ӵļ����������ữ����������
����⣺��1�����ݹ�����д��������Ĺ��ɣ�Mg9FeSi5O20�����������ʽ�ɱ�ʾΪ9MgO?FeO?5SiO2���ʴ�Ϊ��9MgO?FeO?5SiO2��
��2����ӦNaOH��aq��+CO2��g��=NaHCO3��aq����һ���ؼ�С�ķ�Ӧ��Ҫʹ���Է����У�������H��0���ʴ�Ϊ����H��0��
��3����������ͼ����̼ʱ��Ҫ��Ӧ�ķ���ʽΪNaOH��aq��+CO2 ��g��=NaHCO3 ��aq������ҵ�����ȵ�ⱥ��ʳ��ˮ�����ռȱ���ռ����ȡ���̣��ʴ�Ϊ��
 ��
��
��4�������������У�H2NCH2COONa�ͣ�NH4��2CO3���ԺͶ�����̼֮�䷴Ӧ������������̼�����Լ����ʴ�Ϊ��bc��
��5��1 ͼ����ʾ�����Լ����߱仯֪����20min���ܽ�ﵽƽ�⣬���÷�Ӧ�Ƿ��ȣ����£�ƽ�������ƶ������ܽ�Ч�ʽ��ͣ��ʴ�Ϊ��120min���ܽ�ﵽƽ�⣬����Ӧ���ȣ�����ƽ�������ƶ����ܽ�Ч�ʽ��ͣ�
��6���жϲ�Ʒϴ��ֻ��Ҫ����ϴ��Һ�в����������Ӽ��ɣ������ӵļ����������ữ���������������ǣ�ȡ�������һ�ε�ϴ��Һ���������ữ����������Һ������������������ϴ����
�ʴ�Ϊ��ȡ�������һ�ε�ϴ��Һ���������ữ����������Һ������������������ϴ����
��2����ӦNaOH��aq��+CO2��g��=NaHCO3��aq����һ���ؼ�С�ķ�Ӧ��Ҫʹ���Է����У�������H��0���ʴ�Ϊ����H��0��
��3����������ͼ����̼ʱ��Ҫ��Ӧ�ķ���ʽΪNaOH��aq��+CO2 ��g��=NaHCO3 ��aq������ҵ�����ȵ�ⱥ��ʳ��ˮ�����ռȱ���ռ����ȡ���̣��ʴ�Ϊ��
 ��
����4�������������У�H2NCH2COONa�ͣ�NH4��2CO3���ԺͶ�����̼֮�䷴Ӧ������������̼�����Լ����ʴ�Ϊ��bc��
��5��1 ͼ����ʾ�����Լ����߱仯֪����20min���ܽ�ﵽƽ�⣬���÷�Ӧ�Ƿ��ȣ����£�ƽ�������ƶ������ܽ�Ч�ʽ��ͣ��ʴ�Ϊ��120min���ܽ�ﵽƽ�⣬����Ӧ���ȣ�����ƽ�������ƶ����ܽ�Ч�ʽ��ͣ�
��6���жϲ�Ʒϴ��ֻ��Ҫ����ϴ��Һ�в����������Ӽ��ɣ������ӵļ����������ữ���������������ǣ�ȡ�������һ�ε�ϴ��Һ���������ữ����������Һ������������������ϴ����
�ʴ�Ϊ��ȡ�������һ�ε�ϴ��Һ���������ữ����������Һ������������������ϴ����
������������һ����ѧ��������ϵĹ��������⣬�����ڿ��Ե��ȵ㣬ע��֪ʶ��Ǩ�ƺ����Ӧ���ǽ���Ĺؼ����ѶȲ���

��ϰ��ϵ�д�
�����Ŀ