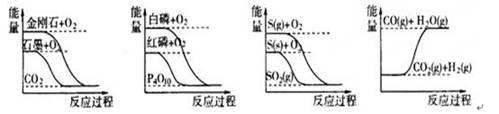
��Ŀ����
��֪2 mol H2(g)ȼ������Һ̬ˮ�ų�������Ϊ571��6 lkJ����2 molH2 (g)��ȫȼ��������̬ˮ�ŵ�����Ϊ483�� 6 kJ������˵����ȷ����
| A��l molH2O (I)���lmolH2O (g)��������ͷ����� |
| B��1 molH2O (g)�ֽ��H2(g)��O2(g)������241�� 8 kJ���� |
| C��2 mol H2(g)��l mol O2(g)����������2 molH2O��1�������� |
| D������2 molH2O (g)�����е�O-H��������483�� 6 kJ���� |
B
���������A��Һ̬ˮ�����̬ˮ��Ҫ��������������B����Ϊ�����2����Ӧ����ЧӦ���෴�ġ�2 molH2 (g)��ȫȼ��������̬ˮ�ŵ�����Ϊ483�� 6 kJ������1 molH2O (g)�ֽ��H2(g)��O2(g)������241��8 kJ��������ȷ��C��2 mol H2(g)ȼ������Һ̬ˮ�ų�������Ϊ571��6 lkJ�����Է�Ӧ��������������������������������D��483�� 6 kJ��2 molH2 (g)��ȫȼ��������̬ˮ����ЧӦ�����ǻ�ѧ���ļ��ܣ�����ѡB��

��ϰ��ϵ�д�
�����Ŀ
 2NH3(g)����H����92��0 kJ��mol��1�������¶��µ�1 mol N2��3 mol H2����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų�������Ϊ(�ٶ�����������û��������ʧ) ( )
2NH3(g)����H����92��0 kJ��mol��1�������¶��µ�1 mol N2��3 mol H2����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų�������Ϊ(�ٶ�����������û��������ʧ) ( )