��Ŀ����
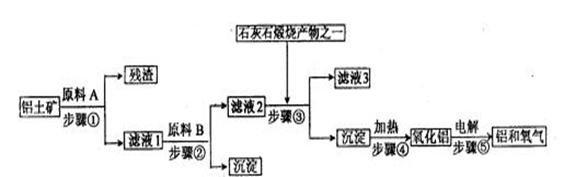
��ҵ����������������Ҫ�ɷ���Al2O3������������Fe2O3��SiO2����ȡұ��������Ҫԭ����������Ȼ����е�⡣������������ͼ��

��1����Һ1��Ҫ�����Թ�����ԭ��B��ԭ��B�Ļ�ѧʽ��______________��д��������йط�Ӧ�����ӷ���ʽ_______________________��_________________________��
��2����Һ3�����ʵ���Ҫ�ɷ���________(�ѧʽ)��д������Һ��һ����;______________________��
��3�����ʡȥ����٣����ܽ��������ֱ�Ӽ���ԭ��B��ʼ�������ջ����������������ʲôӰ�죿
_________________________________��
��4��������������������ĩ����ȼ�������³��������Ӹֹ죬��Ҫ�������˸÷�Ӧ��_____________�����Ҹ÷�Ӧ�����ٶȿ졢�豸���ף�����Ұ����ҵ��
��5������������������н��У����۵��������Ͼ���ʯī������___________���IJ����淴Ӧ�Ľ�����Ҫ���ϲ��䣬Ϊʲô��_____________________________��������
��2����Һ3�����ʵ���Ҫ�ɷ���________(�ѧʽ)��д������Һ��һ����;______________________��
��3�����ʡȥ����٣����ܽ��������ֱ�Ӽ���ԭ��B��ʼ�������ջ����������������ʲôӰ�죿
_________________________________��
��4��������������������ĩ����ȼ�������³��������Ӹֹ죬��Ҫ�������˸÷�Ӧ��_____________�����Ҹ÷�Ӧ�����ٶȿ졢�豸���ף�����Ұ����ҵ��
��5������������������н��У����۵��������Ͼ���ʯī������___________���IJ����淴Ӧ�Ľ�����Ҫ���ϲ��䣬Ϊʲô��_____________________________��������
��1��NaOH��Fe3++3OH-==Fe(OH)3����Al3++4OH-==AlO2-+2H2O
��2��NaHCO3����NaHCO3�ͷۣ��𰸺������ɣ�
��3����NaOHֱ���ܽ��ʹSiO2����Na2SiO3��ͨCO2������H4SiO4��Al(OH)3�������Ӷ����Ⱥ����ɵ�
Al2O3�������SiO2
��4�����ȷ�Ӧ
��5�������������ɵ�O2��ʯī��Ӧ������CO2�Ӷ�������
��2��NaHCO3����NaHCO3�ͷۣ��𰸺������ɣ�
��3����NaOHֱ���ܽ��ʹSiO2����Na2SiO3��ͨCO2������H4SiO4��Al(OH)3�������Ӷ����Ⱥ����ɵ�
Al2O3�������SiO2
��4�����ȷ�Ӧ
��5�������������ɵ�O2��ʯī��Ӧ������CO2�Ӷ�������

��ϰ��ϵ�д�
�����Ŀ