��Ŀ����
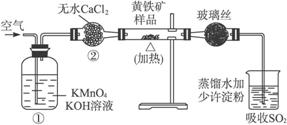
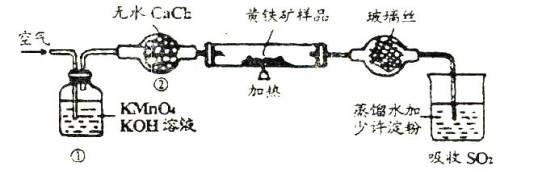
��ͼ��ijʵ���Ҳⶨ����������Ԫ�ص�����������װ�ã�
ʵ��ʱ�����²�����
A.���Ź�����Ʒ���ȵ�800��850 �档
B.��ȡ��ϸ�Ļ�������Ʒ��
C.���Ӻ�ȫ��������������������ԡ�
D.����ƷС�ķ���Ӳ�ʴŹ��в���
E.�Ծ�����ٶȲ��Ϲ��������
F.�ñ�����Һ�ζ������۵�SO2ˮ��Һ��
��1����ȷ��ʵ�鲽��Ĵ����ǣ���A��B��������ű�ʾ��_______________��
��2��װ�âٵ�������_______________��װ�âڵ�������_______________��
��3�����SO2ˮ��Һ��Ӧ�����ӷ���ʽ��_____________________________________��
��4������______________________________�����жϵζ��Ѵﵽ�յ㡣
��5����ͬѧ��Ϊ������װ�ò���Ļ���������Ԫ�ص���������ͨ����ƫ�ͣ����Ƿ�ͬ��ù۵�_______________����ǡ�������˵�������Ҫ����_______________________��
��6���ٶ������е���ȫ��ת��ΪSO2����ȫ����ˮ���ա����Ƶÿ���������Ϊ0.090 0 g���ζ����е���Һ�ij�����Ϊ1.10 mL��ĩ����Ϊ25.60 mL��������Һ��Ũ��Ϊ0.050 0 mol��L-1����û�������Ʒ����Ԫ�ص���������Ϊ_______________��
��1��CBDEAF
��2����ȥ������CO2���������弰��ԭ������?
��ȥˮ����
��3��I2+SO2+2H2O====4H++![]() +2I-
+2I-
��4����Һǡ�ó���ɫ�Ұ���Ӳ���ɫ
��5���ǡ����������巴Ӧ��������ѷ�Ӧ��ȫ����ȼ�ղ���֣�������ˮ��SO2���ֱ�O2������
��6��43.6%
�������������⣬�ⶨ����������Ԫ�ص�����������Ӧ�ⶨ������ȼ�պ����ɵĶ�����������ʵ�����Ҫ������һ�㣬Ӧ��֤���¼��㣺��������ȼ����ȫ�������ɵĶ�������ȫ����ˮ���գ������õ���Һ�ζ������۵�SO2ˮ��Һ֮ǰ��һ��Ҫȷ�����������ʡ�������ͨ����ṩ�����Ŀ����ж�����̼���������弰ˮ����һ��Ҫ�������������ն�������Ĺ�����Ҫ�����ճ����ܿ��ܽ����������Ū������Щ���⣬�Ͳ��ѻش����ϸ�С�⡣
�ڣ�6���ʣ�I2![]() SO2
SO2![]() S
S
1 mol 32 g?
0.024 5��0.05 0.0392 g
W(S)=![]() ��100%=43.6%
��100%=43.6%


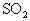 ˮ��Һ��
ˮ��Һ��
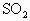 ˮ��Һ������Ӧ�Ļ�ѧ����ʽ��_________��
ˮ��Һ������Ӧ�Ļ�ѧ����ʽ��_________��