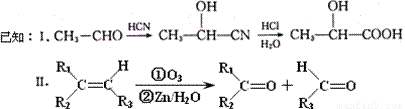
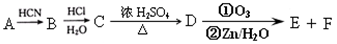��Ŀ����
����һ����Һ�����п��ܺ���Mg2+��Al3+��Fe2+��Cu2+��NH4+��������һ�ֵ���ɫ���岢�����ܽ�ʱ���д̼�����ζ������ų��Ͱ�ɫ�������ɣ����뵭��ɫ��������ʵ����������꣩�������ij����Ͳ�����������ʵ����������꣩�Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
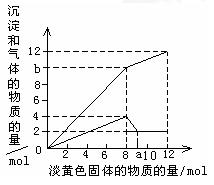
���յİ�ɫ�����ijɷ��ǣ��ѧʽ�� �����������
��2����ȷ������Һ�п��ܺ������������е���Щ���Ӳ���������Ӧ�����ʵ���������±������Բ�������
| ���ܺ��е����� �������ӷ��ţ� | |||||
| ��Ӧ�����ʵ��� |
��4��ͼ��a���ֵΪ
��5��ͼ��b���������ɳɷ��ǣ��ѧʽ�� �������ʵ���֮����
��1��Mg(OH)2��1�֣�������þ������ǿ������������������������������ǿ���2�֣�
��2��
��3�� 9
��4��NH3 O2 ; 3:2
����:
��

��ϰ��ϵ�д�
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
�����Ŀ