��Ŀ����
��ˮ�ĵ���ƽ��������ͼ��ʾ
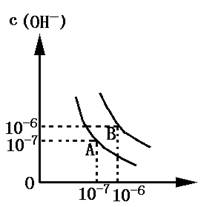
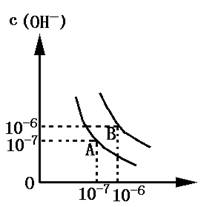
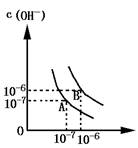
��1������A���ʾ25��Cʱˮ�ڵ���ƽ��ʱ������Ũ�ȣ����¶�������100��Cʱ��ˮ�ĵ���ƽ��״̬ΪB�㣬���ʱˮ�����ӻ���_______���ӵ�________��
��2����pH=8��Ba(OH)2��Һ��pH=5��ϡ�����ϣ�������100��C���£���ʹ�����ҺpH=7 ����Ba(OH)2������������Ϊ____________��
��3����֪20��Cʱ����H2S��Һ��c(H2S) = 0.1mol�� L-1��H2Sһ�����볣��ԼΪ1��10-7�� �������볣��ԼΪ7 ��10-15�����ʱ��Һ��c(H+)=________��
��2����pH=8��Ba(OH)2��Һ��pH=5��ϡ�����ϣ�������100��C���£���ʹ�����ҺpH=7 ����Ba(OH)2������������Ϊ____________��
��3����֪20��Cʱ����H2S��Һ��c(H2S) = 0.1mol�� L-1��H2Sһ�����볣��ԼΪ1��10-7�� �������볣��ԼΪ7 ��10-15�����ʱ��Һ��c(H+)=________��
��1��10-14 ��10-12
��2��2��9
��3��1��10-4mol��L-1
��2��2��9
��3��1��10-4mol��L-1

��ϰ��ϵ�д�
�����Ŀ