��Ŀ����
����ȼ���Ҵ���ҵ��ʮһ�塱�滮����2010�꣬�Ҵ����ͽ�ռ�й�������������һ�����ϣ��Ҵ�������Ϊһ���������ȼ�ϣ������ҹ��Ĺ��ұ����Ҵ���������90%����ͨ������10%��ȼ���Ҵ����Ͷ��ɡ�����Ӱ����������ʻ���ܣ��������к�������ŷ�����(1)д���Ҵ���ȫȼ�յĻ�ѧ����ʽ��______________________________________________��
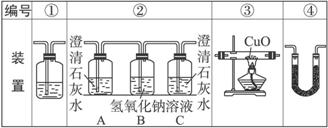
(2)�Ҵ�ȼ��ʱ����������㣬���ܻ���CO���ɡ�����ͼװ����֤�Ҵ���ȼ�ղ�������CO��CO2��H2O��Ӧ���Ҵ���ȼ�ղ�������ͨ��(�����������ҵ�˳����װ�ñ��)__________��

(3)ʵ��ʱ�ɹ۲쵽װ�â���Aƿ��ʯ��ˮ����ǡ�
Aƿ��Һ��������_______________________________________________________��
Bƿ��Һ��������_______________________________________________________��
Cƿ��Һ��������_______________________________________________________��
(4)װ�â۵�������____________________________��װ�â�����ʢ�Լ���______________________��Һ��������______________________________________��
(5)װ�â�����ʢ�Ĺ���ҩƷ��_________________����������֤�IJ�����_______________��
(6)β���Ĵ���������______________________________________________________��
(1)C2H5OH+3O2![]() 2CO2+3H2O
2CO2+3H2O
(2)�ܢڢۢ�
(3)��֤CO2���� ��ȥ��������е�CO2 ֤��CO2�ѱ�����
(4)��CO������CO2 ����ʯ��ˮ ������CO��CuO��Ӧ�����ɵ�CO2���Ӷ���֤ԭ��������CO
(5)��ˮ����ͭ H2O
(6)��ȼ

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�����ʮ�ߴ�ָ��������ǿ��Դ��Դ��Լ����̬������������ǿ�ɳ�����չ��������ֽ�Լ��Դ�ͱ��������Ļ������ߣ���չ������ҵ����
(1)�����й���������������Ҫ�����________��
| A����úҺ�������������ȼ�ϵ�ȼ��Ч�� |
B����װ����β����ת��װ�ã�ʹ֮��Ӧ��4CO��2NO2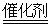 4CO2��N2 4CO2��N2 |
| C�������ƹ��Ҵ����͵�ͬʱ���о�����̫������������ȼ�յ������ |
| D����ˮ�����硢�������硢���ܷ���ͷ���������Ҫ������չ�������� |
C(s)��H2O(g)
 CO(g)��H2(g)��C(s)��H2(g)��CO(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)��H2(g)��C(s)��H2(g)��CO(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��C(s)��O2(g)===CO2(g) ��H����393.5 kJ/mol�� ��
H2(g)��
 O2(g)===H2O(g) ��H����242.0 kJ/mol ��
O2(g)===H2O(g) ��H����242.0 kJ/mol ��CO(g)��
 O2(g)===CO2(g) ��H����283.0 kJ/mol�� ��
O2(g)===CO2(g) ��H����283.0 kJ/mol�� ����ش�
�ٸ����������ݣ�д��C(s)��H2O(g)��Ӧ���Ȼ�ѧ����ʽ��_____________________________________.
��ˮú������������������ȼ�ϣ�Ҳ����Ҫ���л�����ԭ�ϣ�CO��H2��һ�������¿��Ժϳɣ�a.�״���b.��ȩ��c.���d.���ᣮ�Է�����CO��H2��1��1������Ȼ�Ϸ�Ӧ���ϳ�����________(�����)����ʱ���������㡰��ɫ��ѧ����Ҫ����ȫ����ԭ���е�ԭ�ӣ�ʵ�����ŷţ�

 4CO2��N2
4CO2��N2 CO(g)��H2(g)��C(s)��H2(g)��CO(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)��H2(g)��C(s)��H2(g)��CO(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ�� O2(g)===H2O(g) ��H����242.0 kJ/mol ��
O2(g)===H2O(g) ��H����242.0 kJ/mol ��