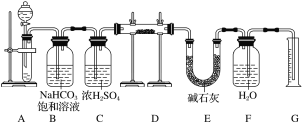
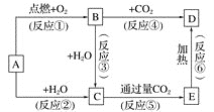
��Ŀ����
����Ŀ����ˮ�Ǿ����Դ���⣬���Խ����ۺ����á��Ӻ�ˮ����ȡ��ˮ��ʳ�κ���Ĺ�����ͼ��
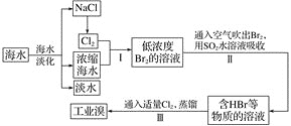
��1���������Br2�Ļ�ѧ��Ӧ�����ӷ���ʽΪ_______��
��2���������SO2ˮ��Һ����Br2��ʹ������ת��Ϊ�������Դﵽ������Ŀ�ģ��䷴Ӧ�Ļ�ѧ����ʽΪ____���ڸ÷�Ӧ�У���������____���ѧʽ��������Ӧ������2mol HBr��������___mol SO2��
��3������������Ӧ���жϳ�SO2��Cl2��Br2����������������ǿ������˳����_______��
���𰸡�Cl2+2Br-=2Cl-+Br2 Br2+SO2+2H2O=H2SO4+2HBr Br2 1 Cl2��Br2��SO2
��������
��1��������ǰ�����ͨ��Ũ����ˮ�У��Ѻ�ˮ�е�Br-����ΪBr2�����ӷ���ʽΪ��Cl2+2Br-=2Cl-+Br2��
��2��������У�SO2ˮ��Һ��Br2����������ԭ��Ӧ��ʹ������ת��Ϊ�����ᣬͬʱSO2ת�������ᣬ��Ӧ�Ļ�ѧ����ʽΪBr2+SO2+2H2O=H2SO4+2HBr���ڸ÷�Ӧ�У�Br2�õ����ӣ��������������ݻ�ѧ����ʽ������Ӧ������2mol HBr��������1mol SO2��
��3�����ݷ�ӦCl2+2Br-=2Cl-+Br2���жϳ�Cl2��������ǿ��Br2���ٸ��ݷ�ӦBr2+SO2+2H2O=H2SO4+2HBr��֪Br2��������ǿ��SO2������SO2��Cl2��Br2����������������ǿ������˳����Cl2��Br2��SO2��

 ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д�