��Ŀ����
����Ŀ��һ�������£�Fe��HCN��K2CO3�ɷ�����ӦFe+6HCN+2K2CO3=K4Fe(CN)6+H2��+2CO2��+2H2O����ش��������⣺
(1)�������Ķѻ���ʽΪ___________������λ��Ϊ___________��
(2)HCN���ӵĽṹʽΪ___________��д��һ����CN����Ϊ�ȵ�����������ӣ�___________��
(3)����NH3___________(����>����<������=")NF3��ԭ����___________��
(4)K4Fe(CN)6���������ӵĺ�������Ų�ʽΪ___________��
(5)C��N��O��H�ĵ�һ��������С�����˳��Ϊ___________��
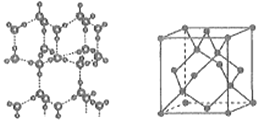
(6)���ľ���ṹģ����ͼ��ʾ���侧���ṹ(��ͼ��ʾ)����ʯ�ľ����ṹ���ƣ�ˮ���Ӽ��������������һ�������к���___________���������NA��ʾ�����ӵ�������ֵ��������ļ���Ϊdnm�������ܶ���=___________g��cm��3(�ú���d��NA�Ĵ���ʽ��ʾ)��
���𰸡����������ѻ� 8 ![]() C22- �� N������sp3�ӻ�������һ�Թµ��Ӷԣ����縺��F��N����NF3�гɼ����Ӷ�Զ������ԭ��N���ų�����С�����ǽ�С 1s22s22p63s23p63d6��[Ar]3d6 C��H��O��N 16
C22- �� N������sp3�ӻ�������һ�Թµ��Ӷԣ����縺��F��N����NF3�гɼ����Ӷ�Զ������ԭ��N���ų�����С�����ǽ�С 1s22s22p63s23p63d6��[Ar]3d6 C��H��O��N 16 ![]()
��������
��1���������Ľ��ܶѻ���ʽΪ���������ѻ�������Feԭ���붥��8��Feԭ�����ڣ�����λ��Ϊ8��
��2��HCN̼�γ�2��������2��������HCN���ӵĽṹʽΪ![]() ����CN-��Ϊ�ȵ��������������C22-��
����CN-��Ϊ�ȵ��������������C22-��
��3������N������sp3�ӻ�������һ�Թµ��Ӷԣ����縺��F��N����NF3�гɼ����Ӷ�Զ������ԭ��N���ų�����С�����ǽ�С������NH3��NF3���Dz�ͬ��
��4�������K4Fe(CN)6���������ӵĵ����Ų�ʽΪ1s22s22p63s23p63d6��[Ar]3d6��
��5��C��N��O����ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ����ڢ�A�壬�ڢ�A��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ��������ǵĵ�������С�����˳����C<O<N��ͬʱH���ڵڶ����ڣ�Ҳ����C��N��Oͬ���壬����ֱ���жϣ��ɲο��㾦��ͼ��H��C��O֮�䣬�������մ�C<H<O<N��
��6��һ��������ˮ������Ϊ8��1/8+6��1/2+4=8����������У�ÿ����ˮ���Ӽ���һ�������ƽ������ÿ��ˮ�����а����һ��ˮ��������Χ���ĸ�ˮ�����������ϣ���1mol������2mol�������һ����������8��2=16����������������Ϊdnm�������ܶ�(g��cm-3)����ʽΪ![]() ��
��

����Ŀ����Cl2���������л���ʱ�����HCl���������·�Ӧ��ʵ���ȵ�ѭ�����á�4HCl(g)+O2(g)=2Cl2(g)+2H2O(g)+Q(Q>0)��2L�ܱ������н��и÷�Ӧ���ڲ�ͬʱ����ʵ���������±���
���� ���ʵ��� (mol) ʱ�� | HCl(g) | O2(g) | H2O(g) | Cl2(g) |
0min | 4 | 1 | 0 | 0 |
2min | 1.2 | 0.3 | 1.4 | |
3min | 1.2 | 0.3 | 1.4 | |
4min | 1.0 | 0.35 | 1.5 |
(1)����0~2min��Cl2��ƽ����������___________________�� �÷�Ӧ���ʵ��¶ȷ�Χ��380~440�棬ѡ����¶ȷ�Χ���ܵ�ԭ���ǣ��ټӿ췴Ӧ���ʣ���_______________________________��
(2)��ҵ���ô�������ⷴӦ�����е�����ѹǿ����ѹǿ���ֲ���ʱ�����淴Ӧ�Ѵ�ƽ�⡣��ͨ�����ѹǿ�жϷ�Ӧ�Ƿ�ﵽƽ��״̬��������__________________________��
(3)ʵ��ʱ����3~4min֮��ı���ijһ�������ƽ���ƶ�����������˵��ƽ����____�ƶ�(����������������)���ﵽ��ƽ�����ԭ�����ƽ�ⳣ��_______(���������������С������������)��
(4)Cl2Ҳ�������Ʊ����;�ˮ����������(Na2FeO4)����ƽ�Ʊ���Ӧ�Ļ�ѧ����ʽ___Fe(NO3)3+___NaOH+___Cl2��___Na2FeO4+___NaNO3+___NaCl+___H2O����Ӧ����3.36L Cl2(��״��)����ת�Ƶ��ӵ���Ŀ��________��
(5)��Na2FeO4ɱ���������ŵ��ǻ�ԭ������о�ˮ���ã�ȱ���ǻ�ԭ����������ˮ����ɸ�ʴ����ɸ�ʴ��ԭ����________________________________________��
����Ŀ����֪���Ҷ����׳Ʋ���(�ṹ��ʽΪHOOCCOOH���ɼ�дΪH2C2O4)��25��ʱ������Ƶ�Ksp=4.0��108��̼��Ƶ�Ksp=2.5��109���ش��������⣺
(1)25��ʱ��20mL̼��Ƶı�����Һ����μ���8.0��104mol/L�IJ������Һ20mL���ܷ��������________(������������������)��
(2)����KMnO4��Һ�������(H2C2O4)��Һ��Ӧ��ij̽��С�����÷�Ӧ��������Һ��ɫ��ʧ�����ķ������о�Ӱ�췴Ӧ���ʵ�������
I��ʵ��ǰ������Ũ��Ϊ0.1000molL1����KMnO4����Һ�ζ�δ֪Ũ�ȵIJ��ᡣ
�ٵζ������в����ζ��ܵ�ͼʾ��ȷ����________(����)��

���жϵζ��յ�ķ�������________��
������������KMnO4����Һʱ����������ƿ�Ŀ̶��ߣ���ʹ��õIJ�����ҺŨ��__________(����ƫ��������ƫ��������������)��
����ͨ���ζ�ʵ��õ�������Һ��Ũ��Ϊ0.2000molL1���øò�����Һ���±����к���ʵ��(ÿ��ʵ�������Һ��������Ϊ8mL)��
ʵ���� | �¶� (��) | �������� (g) | ���Ը��������Һ | ʵ��Ŀ�� a��ʵ��1��2̽�� | |
���(mL) | Ũ��(molL1) | ||||
1 | 25 | 0.5 | 4 | 0.1000 | |
b��ʵ��1��3̽����Ӧ��Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ�죻c��ʵ��1��4̽�������Ը÷�Ӧ���ʵ�Ӱ�졣 | |||||
2 | 50 | 0.5 | 4 | 0.1000 | |
3 | 25 | 0.5 | 4 | 0.0100 | |
4 | 25 | 0 | 4 | 0.1000 | |
��д������a��Ӧ��ʵ��Ŀ��________��
�ݸ�С��ͬѧ��ʵ��1��3�ֱ����������ʵ�����������ʵ������(�ӻ�����ȿ�ʼ��ʱ)��
ʵ���� | ��Һ��ɫ����ʱ��(min) | ||
��1�� | ��2�� | ��3�� | |
1 | 14.0 | 13.0 | 11.0 |
3 | 6.5 | 6.7 | 6.8 |
�����������ݺ�ó���������������ͬʱ�����Ը��������Һ��Ũ��ԽС����ɫʱ���Խ�̣�����Ӧ���ʾ�Խ�����Ľ�����ijͬѧ��Ϊ��С����̽����Ӧ��Ũ�ȶ�����Ӱ������ʵ�鷽������д����������Ӷ��õ��˴����ʵ�������������Ľ���ʵ�鷽��______��




