��Ŀ����
B���������ƹ����ܽ���ˮ����Һ�¶����ߣ�
C��������Һʱ��ˮ�����˿̶ȣ�ʵ��ʧ�ܣ�Ӧ�������ƣ�
D�����ƺõ�����������Һ��Ӧת�������������Լ�ƿ�����ϱ�ǩ��ϴ������ƿ��
B��NaOH�������ձ����ܽ��Ӧ��ȴ��������ת����Һ����B����
C��������Һʱ����ˮ�����̶ȣ������½������ƣ���C����
D����Һ������ϣ���������ת�������������Լ�ƿ�����ϱ�ǩ��ϴ������ƿ�����ϲ���Ҫ����ȷ��
��ѡD��

2005�꽭��������Ⱦ�������أ����귢��Ƶ��Ϊ34.1����������6.2���ٷֵ㣬ÿ�������о���һ���������ꡣijУ�о���ѧϰС����Ա��п����ж�����������![]() �����вⶨ�����������Ͽɲ������·�����
�����вⶨ�����������Ͽɲ������·�����
ʵ��ԭ���������еĶ��������ü�ȩ��Һ���պ������ȶ����Ǽ�����ӳɻ�����ټ�����������ʹ�ӳɻ�����ֽ⣬�ͷų����������븱õ�屽������ȩ���ã������Ϻ�ɫ�����������ɫ��dz���÷ֹ��ȼ���577nm�����вⶨ��
�ֹ��ȼ������÷ֹ��ȷ������ʽ��ж��������Է��������������ֹ��ȷ�����ͨ���ⶨ�����������ض�����ʱ������նȣ��Ը����ʽ��ж��ԺͶ����������乤��ԭ�����Լ��ù�ʽ��ʾΪ![]() ������AΪ����ȣ�KΪһ�������µij�����cΪ��ҺŨ�ȡ�
������AΪ����ȣ�KΪһ�������µij�����cΪ��ҺŨ�ȡ�

ʵ�鲽�裺
��1��������
�������Ǹ���ȤС���Ա����Բ����ĵص��ʱ���кν��飺
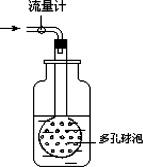
����ͼװ�ò�����ͨ��50L����������Һ����100mL����ƿ������������Һ������ϴ������װ�ã��ϲ�ϴ��Һ������ƿ�У����ݱ��á��ö�����ݶ����õ��ܵ�ԭ����
��2���������![]() ���������ߵĻ��ƣ���6֧25mL��ɫ���У���ÿ������25
���������ߵĻ��ƣ���6֧25mL��ɫ���У���ÿ������25![]() ��������ı�Һ�����±��еı�
��������ı�Һ�����±��еı�![]() ����ϵ�С�
����ϵ�С�
���������ϵ��
| �ܺ� | 0 | 1 | 2 | 3 | 4 | b |
| ��Һ��mL | 0 | 0.20 | 0.40 | 0.60 | 0.80 | 1.00 |
| ����Һ��mL | 10.00 | 9.80 | 9.60 | 9.40 | 9.20 | 9.00 |
| ������������ | 0 | 5��00 | 10.00 | 15��00 | 20.00 | 25.00 |
��10mL��ɫ����ˮΪ�αȣ��ⶨ��������ȣ�ʵ�������¡�
| �� �� | 0 | 1 | 2 | 3 | 4 | 5 |
| ����� | 0 | 0.20 | 0.40 | 0.60 | 0.80 | 1.00 |
�������ֵΪ�����꣬������������![]() ��Ϊ�����꣬���Ʊ����ߡ�
��Ϊ�����꣬���Ʊ����ߡ�
��3��ȷ��ȡ����ƿ����Һ10mL���˱�ɫ����������ֵΪ0.24��������ж���������Ϊ ![]() ���ҹ����������������ж�ÿ�ο��������ⶨ��
���ҹ����������������ж�ÿ�ο��������ⶨ��![]() �����Ũ����ֵ��m9��m3����һ������0.15����������0.50����������0.70������п���Ϊ ������
�����Ũ����ֵ��m9��m3����һ������0.15����������0.50����������0.70������п���Ϊ ������
��4���ס�������ͬѧ�ⶨ������ϴ��Է�������ͬѧ�ⶨ������![]() ����ƫС�Ŀ���ԭ����������ҩƷ��װ�þ������⣩
����ƫС�Ŀ���ԭ����������ҩƷ��װ�þ������⣩
��5������������շ��������������Ҫ��ʩ��