��Ŀ����
����Ŀ���������������������ʴ��ÿ����ʴ����ʧ�ĸֲ�ռ���������������ķ�֮һ��
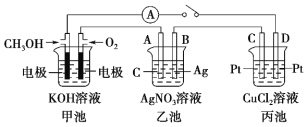
(1)������ʴ��Ҫ��������ʴ����д���ø�ʴ�����еĵ缫��Ӧʽ������________������________��
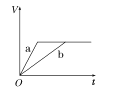
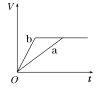
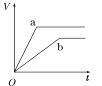
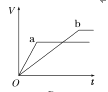
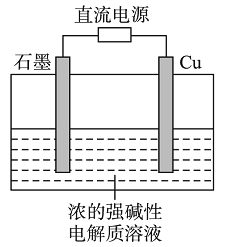
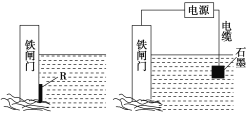
(2)Ϊ�˼���ijˮ�����բ�ű���ʴ�����ʣ����Բ���ͼ����ʾ�ķ��������к�������բ���ϵĹ������R���Բ���________(�����)��
A��ͭ B����
C��п D��ʯī
(3)ͼ����ʾ�ķ���Ҳ���Լ�����բ�ŵĸ�ʴ���ʣ�������բ��Ӧ��������ֱ����Դ��________����
ͼ�ס�������������������ͼ��
���𰸡�2Fe��4e��===2Fe2�� O2��2H2O��4e��===4OH��C��
��������
����Fe����������ʴ��������2Fe-4e-2Fe2+��������O2+2H2O+4e-4OH-����������������������������������������ӵ�����������������

��ϰ��ϵ�д�
�����Ŀ