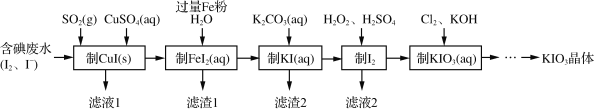
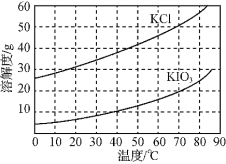
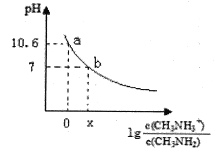
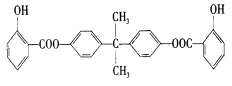
��Ŀ����
����Ŀ��������̼����ɴ�����Ⱦ����Ҫ�к�����֮һ����������β�������ķ���֮һ�Dz������������ϴ�������
��һ������NaOH��Һ����SO2��2NaOH+SO2��Na2SO3+H2O
�ڶ����������õ�Na2SO3��Һ����ʯ�ҷ�Ӧ��Na2SO3+CaO+H2O��CaSO3��+2NaOH
���������գ�
(1)������������Ӧ���漰�Ķ�����Ԫ���У�ԭ�Ӱ뾶��С�����˳����__________��
(2)����������ͬ��Ԫ�أ�����Ԫ�ص�ԭ�����������Ų��ɱ�ʾΪ__________��д��һ���ܱȽ���Ԫ�غ���Ԫ�طǽ�����ǿ���Ļ�ѧ��Ӧ����ʽ��_______________
(3)NaOH�ĵ���ʽΪ_____________��
(4)���ڵ�һ������SO2��õ���NaOH��Na2SO3�����Һ�У�����������ˮ������Һ��Ϊ��ɫ������Na2SO4��NaBr��д��������Ӧ�Ļ�ѧ����ʽ��_____________
(5)���������ϴ��������ŵ���__________��____________������д���㣩
���𰸡�H<O<S<Na ns2np4 2H2S+O2 ![]() 2S+2H2O
2S+2H2O ![]() 2NaOH+Na2SO3+Br2��Na2SO4+2NaBr+H2O NaOH����SO2��Ч�ʸ� NaOH��ѭ�����ã���ʯ�Ҽ۸�ϵͣ��ɱ���
2NaOH+Na2SO3+Br2��Na2SO4+2NaBr+H2O NaOH����SO2��Ч�ʸ� NaOH��ѭ�����ã���ʯ�Ҽ۸�ϵͣ��ɱ���
��������
(1)����������Ӧ���漰�Ķ�����Ԫ�����⡢�����ơ�����Ԫ�������ɵ�֪ʶ���
(2)���ݹ���ԭ����дO��SԪ�ص�ԭ�����������Ų�ʽ���ǽ���ǿ���ܽ��ǽ�������Ԫ���û��������ɴ���д��ѧ����ʽ��
(3)NaOH�����ӻ����Na+��OH-ͨ�����Ӽ���ϣ�
(4)���ռ��Na2SO3�����Һ�м���������ˮ������Һ��Ϊ��ɫ��˵������������ӱ��嵥������Ϊ��������ӣ�
(5)�����������������Ʒ�Ӧ�����ʸߣ�ԭ���������ƿ���ѭ�����ã�����ʯ�ҵļ۸�͡�
(1)����������Ӧ���漰�Ķ�����Ԫ�����⡢�����ơ�������Ԫ�أ��ƺ���ԭ�Ӻ������������Ӳ㣬����ԭ�Ӻ���ֻ��2�����Ӳ㣬��ԭ�Ӻ���ֻ��1�����Ӳ㣬����ԭ�Ӻ�����Ӳ���Խ�࣬ԭ�Ӱ뾶Խ��ԭ�Ӻ�����Ӳ�����ͬʱ��ԭ������Խ��ԭ�Ӱ뾶��ԽС�����뾶��С�����˳���ǣ�H<O<S<Na��
(2)O��Sԭ�Ӻ�������㶼����6�����ӣ����ݹ���ԭ������֪����Ԫ�ص�ԭ�����������Ų�ʽΪns2np4���ǽ���ǿ��Ԫ�صĵ����ܽ��ǽ�������Ԫ�صĵ��ʴӻ��������û���������������������Ӧ���ɵ�����Ӧ�Ļ�ѧ����ʽΪ��2H2S+O2 ![]() 2S+2H2O��
2S+2H2O��
(3)NaOH�����ӻ����������Na+��������OH-֮��ͨ�����Ӽ���ϣ�OH-��H��Oԭ��֮��ͨ�����ۼ���ϣ���NaOH�ĵ���ʽΪ��![]() ��
��
(4)��NaOH��Na2SO3�����Һ�м���������ˮ������Һ��Ϊ��ɫ��˵��SO32-���ӱ��嵥������ΪSO42-���ӣ���Ӧ�Ļ�ѧ����ʽΪ��2NaOH+Na2SO3+Br2��Na2SO4+2NaBr+H2O��
(5)�����������������Ʒ�Ӧ�����ʸߣ�ԭ���������ƿ���ѭ�����ã�����ʯ�ҵļ۸�ͣ��Ӷ��ɽ��������ɱ���

 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�