��Ŀ����
��2013?����ģ�⣩ij��Һ��ֻ���ܺ������������еļ��֣���������Һ�к��Ľ��ٵ�H+��OH-��Na+��NH4+��SO42-��CO32-��NO3-��ȡ200mL����Һ����Ϊ����������ݷֱ�������ʵ�飮ʵ��1����һ�ݼ����������ռ���ȣ������������ڱ�״����Ϊ224mL��ʵ��2���ڶ����ȼ������������ᣬ�������ټ�������BaCl2��Һ���ù���2.33g������˵����ȷ���ǣ�������
������ʵ��1����һ�ݼ����������ռ���Ȼ��������֤������NH4+��ʵ��2���ڶ����ȼ������������ᣬ����������CO32-���ټ�������BaCl2��Һ���ù���2.33g��֤������SO42-��
����⣺����ʵ��1����һ�ݼ����������ռ���ȣ����������224mL��֤������NH4+�������ʵ���Ϊ0.01mol��
ʵ��2���ڶ����ȼ������������ᣬ��������һ��������CO32-���ټ�������BaCl2��Һ���ù���2.33g��֤��һ������SO42-�������ʵ���Ϊ��
=0.01mol��������Һ�еĵ���غ㣬��һ�����������ӣ��������ӵ�Ũ�ȡ�
=0.1mol/L��
A������Һ��һ������Na+����A����
B������Һ�п϶�����NH4+��S042-��Na+����B����
C������Һ�п��ܺ���NO3-����C����
D��������Һ��NH4+���ʵ���Ϊ0.01mol��SO42-���ʵ���Ϊ0.01mol�����ݵ���غ���c��Na+����0.1 mol/L����D��ȷ��
��ѡD��
ʵ��2���ڶ����ȼ������������ᣬ��������һ��������CO32-���ټ�������BaCl2��Һ���ù���2.33g��֤��һ������SO42-�������ʵ���Ϊ��
| 2.33g |
| 233g/mol |
| 0.01mol��2-0.01mol |
| 0.1L |
A������Һ��һ������Na+����A����
B������Һ�п϶�����NH4+��S042-��Na+����B����
C������Һ�п��ܺ���NO3-����C����
D��������Һ��NH4+���ʵ���Ϊ0.01mol��SO42-���ʵ���Ϊ0.01mol�����ݵ���غ���c��Na+����0.1 mol/L����D��ȷ��
��ѡD��
���������⿼���˻����ɷֵļ�����ɴ��⣬�����������ʵ����ʽ��У�ע������֮��ķ�Ӧ�Լ������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
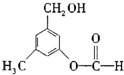 ��2013?����ģ�⣩�л���A�Ľṹ��ʽ��ͼ��ʾ��ijͬѧ������ܾ��еĻ�ѧ���ʽ���������Ԥ�⣬������ȷ���ǣ�������
��2013?����ģ�⣩�л���A�Ľṹ��ʽ��ͼ��ʾ��ijͬѧ������ܾ��еĻ�ѧ���ʽ���������Ԥ�⣬������ȷ���ǣ�������