��Ŀ����
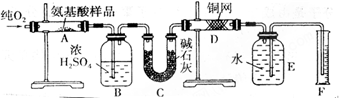
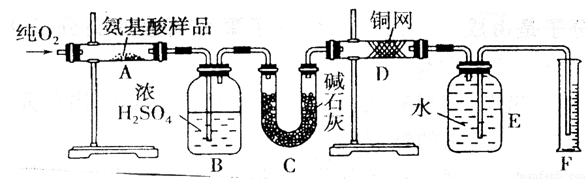
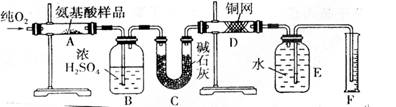
�¹���ѧ�����ϣ������������л���������Ԫ�ض���������������CuO�������������������У����л����������ٶԲ�����з������Ӷ�ȷ���л����ʵ��ʽ����ȡmgij�ְ����ᣨCxHyOzNp���ڴ�������ȫȼ�գ�����CO2��H2O��N2��ʵ������ͼװ���н��У�
��ش��������⣺
��ʵ�鿪ʼʱ����Ҫͨһ��ʱ�����������������__________________________��
�� װ��ͼ����Ҫ���ȵ������У�����ĸ��գ���ͬ��_________������ʱӦ�ȵ�ȼ________���ľƾ��ơ�
��Aװ���з�����Ӧ�Ļ�ѧ����ʽ��______________________________________��
�� Dװ�õ�������______________________________________________��
�� ��ȡN2������ǣ�Ӧע�⣺��____________________________________________��
��_____________________________________________________��
�� ʵ���в��N2�����ΪV mL��������ɱ�״���£���Ϊȷ���˰�����ķ���ʽ������Ҫ���й�������_______________��
A�����ɶ�����̼�������� B������ˮ������
C��ͨ����������� D�����������Է�������
�� ��װ���еĿ���������ȷ�ⶨ��F�еĵ������
�� A��D D
�� CxHyOzNp + ��x + y / 4 �� z / 2��O2 xCO2 + y / 2 H2O + p / 2 N2
�� ����δ��Ӧ����������֤�����ռ��������ǵ���
�� �� ��Ͳ��Һ������ƿ�е�Һ����ͬһˮƽ���� �� �����밼Һ����ͬһˮƽ����
�� A��B��D
����:��