��Ŀ����
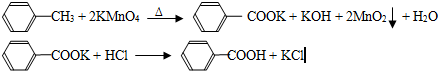
������㷺Ӧ������ҩ�ͻ�����ҵ��ijͬѧ�����üױ���������Ӧ�Ʊ������ᣬ��Ӧԭ����
![]()
![]()
![]() CH3 + 2KMnO4 �� COOK + KOH + 2MnO2 + H2O
CH3 + 2KMnO4 �� COOK + KOH + 2MnO2 + H2O
![]()
![]()
![]() COOK + HCl COOH + KCl
COOK + HCl COOH + KCl
|
|
|
|
|
|
|
|
| |||||
|
|
��֪�������������122���۵�122.4�棬��25���95��ʱ�ܽ�ȷֱ�Ϊ0.3 g��6.9 g�����������л��ﶼ�й̶��۵㡣
��1��������Ϊ ��������Ϊ ��
��2����ɫҺ��A�� �����Լ���A���Լ��� ��������
��
��3���ⶨ��ɫ����B���۵㣬��������115�濪ʼ�ۻ����ﵽ130��ʱ�����������ۣ���ͬѧ�Ʋ��ɫ����B�DZ�������KCl�Ļ�����������·��������ᴿ�ͼ��飬ʵ���������Ʋ���ȷ�����ڴ������ɱ������ݡ�
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ����ɫ����B����ˮ�У������ܽ⣬
| �õ���ɫ�������ɫ��Һ | |
| �� | ȡ������Һ���Թ��У� | ���ɰ�ɫ���� | ��Һ��Cl�� |
| �� | �����ɫ���壬 |
| ��ɫ�����DZ����� |
��4�����Ȳⶨ����ȡ1.220g��Ʒ�����100ml�״���Һ����ȡ25.00ml��Һ���ζ�������KOH�����ʵ���Ϊ2.40��10��3mol����Ʒ�б��������������ļ������ʽΪ ��������Ϊ ��������λ��Ч���֣���
��1����Һ������
��2���ױ�������KMnO4��Һ����ɫ��Һ��ɫ��
��3��
| ��� | ʵ�鷽�� | ʵ������ |
|
| �� | ����ɫ����B����ˮ�У����ȣ��ܽ⣬ ��ȴ������ | �õ���ɫ�������ɫ��Һ | |
| �� | ȡ������Һ���Թ��У����������������ữ��AgNO3��Һ | ���ɰ�ɫ���� | ��Һ����Cl- |
| �� | �����ɫ���壬 ����ʹ���ڻ��������۵㣻 | �۵�Ϊ122.4�� | ��ɫ�����DZ����� |
��4�� [(2.40��10-3��122��4)/1.22]��100%�� 96%

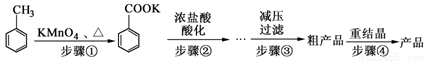
������㷺Ӧ������ҩ�ͻ�����ҵ��ij��ѧС���üױ�����Ҫԭ���Ʊ������ᣬ��Ӧ�������£�
�ױ���������ء�������IJ����������ʼ��±���
|
���� |
�۵�/�� |
�е�/�� |
�ܶ�/g��cm-3 |
��ˮ���ܽ��� |
|
�ױ� |
��95 |
110.6 |
0.8669 |
���� |
|
������� |
121.5��123.5 |
|
|
���� |
|
������ |
122.4 |
248 |
1.2659 |
�� |
��1��������ٵõ�����������ˮ�������л����ˮ�ࡣ�л����� ����ϡ����¡����㣻ʵ������������� ��
��2���������Ũ�����ữ��Ŀ���� ��
��3����ѹ����װ������������������ѹϵͳ�⣬���� �� �����������ƣ���
��4����֪�¶�Խ�ͱ�������ܽ��ԽС����Ϊ�˵õ�����ı����ᾧ�壬�ؽᾧʱ�����¶�Խ��Խ�ã������� ��
��5���ؽᾧʱ��Ҫ���ȹ��ˣ�Ŀ���� ��