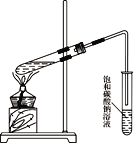
��Ŀ����
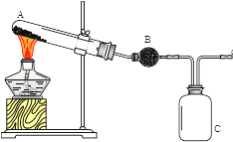
����Ŀ��ij��ѧ��ȤС��������ͼװ����ȡ������̽���������й����ʡ�
���������գ�
��1��Aװ���м���NH4Cl��Ca(OH)2��д������ʱ������Ӧ�Ļ�ѧ����ʽ��___________________________
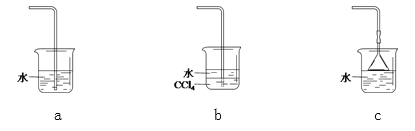
��2������Cװ���ռ�������������ͼ�л���װ���ڵ�����ȷ�����ӷ�ʽ��
_____________
Ϊ��ֹ���������ܳ���ĩ�˿�������__________��ѡ����ţ���
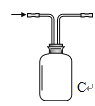
��3����������װ��̽�������Ļ�ԭ�ԣ���װ��C�滻Ϊ��ͼ����d��ͬʱͨ�봿�������������������Ӧ��8NH3+3Cl2��6NH4Cl+N2��

д��װ��C�е�����_______________________
�������NH4Cl��NH4+�ķ�����_______________________
��4��д����ˮ��NH3��H2O�ĵ��뷽��ʽ��________________________
���ʵ��֤����ϡ�Ͱ�ˮʱ��������ƽ���ᷢ���ƶ���_________________________
���𰸡� 2NH4Cl + Ca(OH)2 �� CaCl2 + 2NH3��+2H2O  bc ����ɫ��ȥ���а������� ȡ������ˮ��������������Ũ��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ������֤������NH4+ NH3��H2O
bc ����ɫ��ȥ���а������� ȡ������ˮ��������������Ũ��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ������֤������NH4+ NH3��H2O![]() NH4+ + OH�� ����һ��ȡ��ͬpH�İ�ˮ��NaOH��Һ���ֱ�ϡ����ͬ��������ˮ��pH����NaOH
NH4+ + OH�� ����һ��ȡ��ͬpH�İ�ˮ��NaOH��Һ���ֱ�ϡ����ͬ��������ˮ��pH����NaOH
��������ȡpH=a�İ�ˮ��ϡ��10����ˮ��pH����a-1
��3�֣��������ɣ��ж��գ������Ӧ��ȷ����pH�ⶨ�йأ�
����������1��Aװ���м���NH4Cl��Ca(OH)2������ʱ������Ӧ���ɰ�������ѧ����ʽΪ2NH4Cl + Ca(OH)2 ![]() CaCl2 + 2NH3��+2H2O����2�������ܶ�С�ڿ�����Ӧ���������ſ������ռ�����װ���ڵ�����ȷ�����ӷ�ʽΪ
CaCl2 + 2NH3��+2H2O����2�������ܶ�С�ڿ�����Ӧ���������ſ������ռ�����װ���ڵ�����ȷ�����ӷ�ʽΪ![]() ��Ϊ��ֹ���������ܳ���ĩ�˿������Ӷ��۵�©�������ߵ��ܿڲ������Ȼ�̼�У���ѡbc����3�����ڲ����Ȼ�泥�����װ��C�е������ǻ���ɫ��ȥ���а������ɣ�笠�������������Ϸų��������就������������NH4Cl��NH4+�ķ�����ȡ������ˮ��������������Ũ��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ������֤������NH4+����4����ˮ��NH3��H2O��һԪ������뷽��ʽΪNH3��H2O
��Ϊ��ֹ���������ܳ���ĩ�˿������Ӷ��۵�©�������ߵ��ܿڲ������Ȼ�̼�У���ѡbc����3�����ڲ����Ȼ�泥�����װ��C�е������ǻ���ɫ��ȥ���а������ɣ�笠�������������Ϸų��������就������������NH4Cl��NH4+�ķ�����ȡ������ˮ��������������Ũ��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ������֤������NH4+����4����ˮ��NH3��H2O��һԪ������뷽��ʽΪNH3��H2O![]() NH4++OH��������һˮ�ϰ��ĵ���ƽ���֪ϡ�ʹٽ����룬pH�����仯����ʵ�鷽���ǣ�����һ��ȡ��ͬpH�İ�ˮ��NaOH��Һ���ֱ�ϡ����ͬ��������ˮ��pH����NaOH����������ȡpH=a�İ�ˮ��ϡ��10����ˮ��pH����a-1��
NH4++OH��������һˮ�ϰ��ĵ���ƽ���֪ϡ�ʹٽ����룬pH�����仯����ʵ�鷽���ǣ�����һ��ȡ��ͬpH�İ�ˮ��NaOH��Һ���ֱ�ϡ����ͬ��������ˮ��pH����NaOH����������ȡpH=a�İ�ˮ��ϡ��10����ˮ��pH����a-1��