��Ŀ����
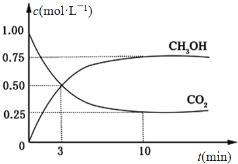
����Ŀ����ͼ����ѧ��ѧ�г������ʵ�ת����ϵ���������ʺͷ�Ӧ������ȥ��
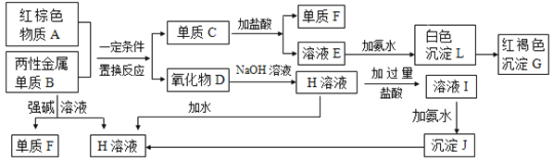
��1��������A�Ļ�ѧʽ��___________��д������D��NaOH��Һ��Ӧ�����ӷ���ʽ______________��
��2��д������B��ǿ����Һ��Ӧ�����ӷ���ʽ_________________��
��3��д���ɳ���J���� H��Һ�����ӷ���ʽ_________________��
��4����ҺE�м��백ˮʱ�������ɰ�ɫ����L��д������L�����ӷ���ʽ___________��
��ɫ����L��Ѹ�ٱ�Ϊ___________ɫ�����ձ�Ϊ���ɫ����G��д��L��ΪG�Ļ�ѧ��Ӧ����ʽ_________________________________��
��5����ҺI�������Ľ���������________________��
���𰸡�Fe2O3 Al2O3+2OH-=2AlO2-+ H2O 2Al+2H2O+2OH-=2AlO2-+3H2�� Al(OH)3+OH-=AlO2-+2H2O Fe2++2NH3H2O=Fe(OH)2��+2NH4+ ���� 4Fe(OH)2+O2+2H2O=4Fe(OH)3 Na+��Al3+
��������
����ɫ����AΪFe2O3��BΪ���Խ������ʣ�A��B�����û���Ӧ����C��D��������D����NaOH��Ӧ��ӦΪAl2O3����BΪAl��CΪFe���������ᷴӦ���ɵ�FΪH2��EΪFeCl2���Ȼ������м��백ˮ���ɰ�ɫ����LΪFe(OH)2��GΪFe(OH)3�����������������Ʒ�Ӧ���ɵ�HΪNaAlO2��ƫ����������������ᷴӦ���ɵ���ҺIΪAlCl3��NaCl�Ļ����Һ��JΪAl(OH)3����϶�Ӧ���ʵ����ʷ������
(1)�����Ϸ�����֪AΪFe2O3��DΪ�����������������������Ʒ�Ӧ����NaAlO2����Ӧ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+ H2O���ʴ�Ϊ��Fe2O3�� Al2O3+2OH-=2AlO2-+ H2O��
(2)����BΪ����Al���������Ƶ�ǿ����Һ��Ӧ����������ƫ�����Σ���Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2H2O+2OH-=2AlO2-+3H2����
(3)����JΪ����������HΪNaAlO2��Al(OH)3�������ԣ�����NaOH��Ӧ����Ӧ�����ӷ���ʽΪAl(OH)3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al(OH)3+OH-=AlO2-+2H2O��
(4)��ҺE�м��백ˮʱ�������ɰ�ɫ����L�����ӷ���ʽΪFe2++2NH3H2O=Fe(OH)2��+2NH4+��Fe(OH)2���ȶ����ɱ���������Fe(OH)3���ɰ�ɫ��Ϊ����ɫ�����ձ�Ϊ���ɫ����Ӧ�Ļ�ѧ����ʽΪ4Fe(OH)2+2H2O+O2=4Fe(OH)3���ʴ�Ϊ��Fe2++2NH3H2O=Fe(OH)2��+2NH4+�����̣�4Fe(OH)2+O2+2H2O=4Fe(OH)3��
(5)IΪAlCl3���Ȼ��ƵĻ����Һ�����еĽ���������Al3+��Na+���ʴ�Ϊ��Al3+��Na+��

 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�