��Ŀ����
��Ҫ�Ļ���ԭ��F��C5H8O4�����������ζ����ͨ���������̺ϳɣ�
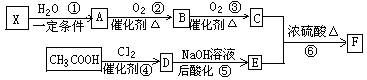
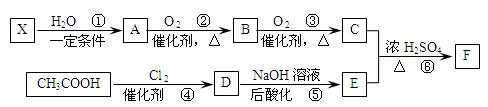
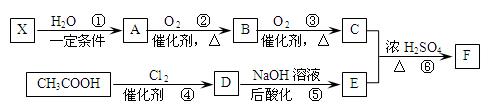
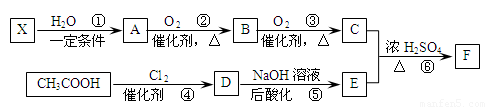
��֪����X��ʯ���ѽ�����Ҫ�ɷ�֮һ������ϩ��Ϊͬϵ��� ����C��E��F������NaHCO3�������塣
����C��E��F������NaHCO3�������塣
��1��ÿ��X�����к� ���Ҽ��� ���м���X�Ӿ۲���Ľṹ��ʽ�� ��
��2��D�����������ŵ������� ��
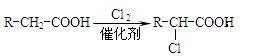
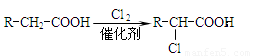
��3����Ӧ�ڵĻ�ѧ����ʽΪ ����Ӧ������ ��
��Ӧ�Ļ�ѧ����ʽΪ ��
��4��F��ͬ���칹��ܶ࣬����һ��ͬ���칹��ֻ����һ�ֹ����ţ������Ի���������¶���ˮ�����������л����ͬ���칹��Ľṹ��ʽ�� ��
��1��8 1 ��2�֣�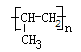 ��2�֣�
��2�֣�
��2����ԭ�ӡ��Ȼ���2�֣�
��3��2CH3CH2CH2OH +O2 ![]() 2CH3CH2CHO+2H2O��2�֣�
2CH3CH2CHO+2H2O��2�֣�
������Ӧ��1�֣�
CH3CH2COOH+HOCH2COOH ![]() CH3CH2COOCH2COOH+H2O ��2�֣�
CH3CH2COOCH2COOH+H2O ��2�֣�
��4��CH3OOCCH2COOCH3��2�֣�

��ϰ��ϵ�д�
�����Ŀ
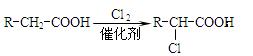 ��
��
 ��
��
 ��
��