��Ŀ����
(��9�֣���֪NaAlO2��HCl��H2O��Al(OH)3����NaCl��ij�����A����
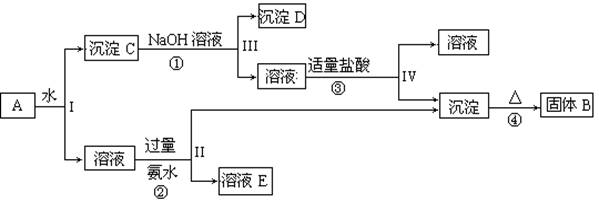
KAl(SO4)2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��
 �ݴ˻ش��������⣺
�ݴ˻ش��������⣺
��1��I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ����� ��
��2������������ͼ��Ӧ��ϵ��д������B��D�������ʵĻ�ѧʽ
����B ������D ��
��3��д���١��ڡ����ĸ���Ӧ�Ļ�ѧ����ʽ�������ӷ�Ӧ��д�����ӷ���ʽ
�� ��
�� ��
�� ��
���𰸡�
��1������
��2��Al2O3 Fe2O3
��3����Al2O3��2OH����2AlO2����H2O
��Al3+��3NH3��H2O��Al(OH) 3����3NH4+
��2Al(OH)
3 Al2O3��3H2O
Al2O3��3H2O
����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �ݴ˻ش��������⣺
�ݴ˻ش��������⣺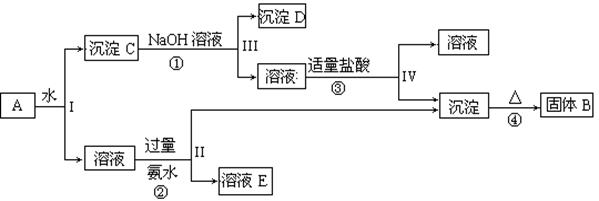 �ݴ˻ش��������⣺
�ݴ˻ش��������⣺ ��
��