��Ŀ����
������Һ�У���Ũ�ȹ�ϵ��ȷ����
| A������NH4����Cl����H����OH�����ӵ���Һ�У�������Ũ��һ���ǣ� c(Cl-)>c(NH4+)> c(H��)> c��OH���� |
| B��pH=6�Ĵ���������ƵĻ����Һ��c(Na+)>c(CH3COO-) |
| C��0.lmol/L��Na2S��Һ�У�c��OH����= c(H��)+ c(HS-)+2 c(H2S) |
| D��pH=3��һ�����pH=11��һ�ּ�������ͺ����Һ��һ����c��OH����=c(H��) |
C
���������A������NH4����Cl����H����OH�����ӵ���Һ�У�������Ũ�Ȼ�������c(NH4+)> c(Cl-)> c��OH����>c(H��)������B��pH=6�Ĵ���������ƵĻ����Һ�У�����ĵ���̶ȴ��ڴ�������ӵ�ˮ��̶ȣ�����c(Na+)<c(CH3COO-)������C��0.lmol/L��Na2S��Һ�У�ˮ���������c��OH����=c(H��)����S2-��ˮ����ˮ���������H��������HS-��H2S�����������غ���ɣ���c��OH����= c(H��)+ c(HS-)+2 c(H2S)����ȷ��D��pH=3��һ�����pH=11��һ�ּ��ǿ��δ֪�����Ե������ͺ����Һ�������δ֪������ѡC��

��ϰ��ϵ�д�
�����Ŀ
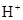 ����c��
����c��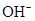 ������ͼ��ʾ��ϵ�������й�˵����ȷ����
������ͼ��ʾ��ϵ�������й�˵����ȷ����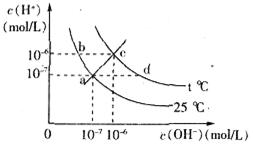
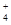 )> c(H��)>c(OH��)
)> c(H��)>c(OH��)
