��Ŀ����
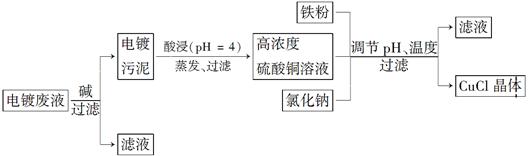
�Ȼ���ͭ(CuCl)�ǰ�ɫ��ĩ������ˮ���������Ҵ����ڿ����лᱻѸ����������ɫ��ʽ�Ρ������Ե�Ʒ�Һ(��Ҫ��Cu2����Fe3��)���Ʊ��Ȼ���ͭ�Ĺ�������ͼ���£�
�������Ӻ�������ҺpH��CuCl��������ҺpH�Ĺ�ϵͼ��ͼ��
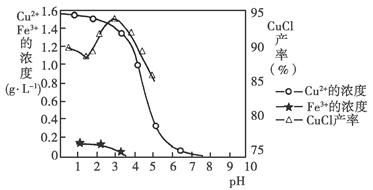
����֪����������Ũ��Ϊ1 mol��L��1ʱ��Fe(OH)3��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ1.4��3.0��Cu(OH)2��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ4.2��6.7����ش��������⣺
(1)���ʱ������Ӧ�����ӷ���ʽ��________������CuCl����ʱ�����pH��________���ҡ�
(2)���ۡ��Ȼ��ơ�����ͭ����Һ�з�Ӧ����CuCl�����ӷ�Ӧ����ʽΪ____________________________��
(3)������CuCl����Ҫ��������ˮ�Ҵ�ϴ�ӣ�����ո��������70�����2 h����ȴ�ܷ��װ��70����ո���ܷ��װ��Ŀ����____________________________________________��
(4)��Ʒ�˳�ʱ������Һ����Ҫ�ֳ���________���������Һ�л�ȡFeSO4��7H2O���壬����Ҫ֪������__________________��
(5)�������ۻ�����������Ҳ�ɵõ��Ȼ���ͭ����д���÷�Ӧ�Ļ�ѧ����ʽ��______________________��Ϊ���CuCl�IJ��ʣ����ڸ÷�Ӧ��ϵ�м���ϡ����Һ������pH��3.5����������Ŀ����__________________________________________��

�������Ӻ�������ҺpH��CuCl��������ҺpH�Ĺ�ϵͼ��ͼ��

����֪����������Ũ��Ϊ1 mol��L��1ʱ��Fe(OH)3��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ1.4��3.0��Cu(OH)2��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ4.2��6.7����ش��������⣺
(1)���ʱ������Ӧ�����ӷ���ʽ��________������CuCl����ʱ�����pH��________���ҡ�
(2)���ۡ��Ȼ��ơ�����ͭ����Һ�з�Ӧ����CuCl�����ӷ�Ӧ����ʽΪ____________________________��
(3)������CuCl����Ҫ��������ˮ�Ҵ�ϴ�ӣ�����ո��������70�����2 h����ȴ�ܷ��װ��70����ո���ܷ��װ��Ŀ����____________________________________________��
(4)��Ʒ�˳�ʱ������Һ����Ҫ�ֳ���________���������Һ�л�ȡFeSO4��7H2O���壬����Ҫ֪������__________________��
(5)�������ۻ�����������Ҳ�ɵõ��Ȼ���ͭ����д���÷�Ӧ�Ļ�ѧ����ʽ��______________________��Ϊ���CuCl�IJ��ʣ����ڸ÷�Ӧ��ϵ�м���ϡ����Һ������pH��3.5����������Ŀ����__________________________________________��
(1)Cu(OH)2��2H��=Cu2����2H2O��3
(2)2Cu2����2Cl����Fe=2CuCl����Fe2��
(3)�ӿ��Ҵ���ˮ����������ֹCuCl����������
(4)Na2SO4��FeSO4
��ͬ�¶��������ƺ������������ܽ��
(5)2CuSO4��Na2SO3��2NaCl��H2O=2CuCl����2Na2SO4��H2SO4
OH���к��˷�Ӧ�е�H����������ƽ�������ƶ������CuCl�IJ��ʡ�����OH��Ũ�ȹ���ʱ��Cu������OH����ϣ�������������ͭ���Ӷ�������CuCl�IJ���
(2)2Cu2����2Cl����Fe=2CuCl����Fe2��
(3)�ӿ��Ҵ���ˮ����������ֹCuCl����������
(4)Na2SO4��FeSO4
��ͬ�¶��������ƺ������������ܽ��
(5)2CuSO4��Na2SO3��2NaCl��H2O=2CuCl����2Na2SO4��H2SO4
OH���к��˷�Ӧ�е�H����������ƽ�������ƶ������CuCl�IJ��ʡ�����OH��Ũ�ȹ���ʱ��Cu������OH����ϣ�������������ͭ���Ӷ�������CuCl�IJ���
(1)�����ɵõ���Ũ�ȵ�����ͭ��Һ�����Է����ķ�ӦΪCu(OH)2��2H��=Cu2����2H2O����ͼ��֪��pH��3ʱCuCl�IJ�����ߡ�(4)��Ӧ�м�����Na������Ӧ��������Fe2����������Һ�ɷ�ΪNa2SO4��FeSO4��(5)�����ۻ���Na2SO3����Ϊ��4��S��ԭCu2����
�㲦�����⿼�黯ѧ�뼼�������鿼���Ի�ѧ�뼼��������������Ѷ��еȡ�
�㲦�����⿼�黯ѧ�뼼�������鿼���Ի�ѧ�뼼��������������Ѷ��еȡ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
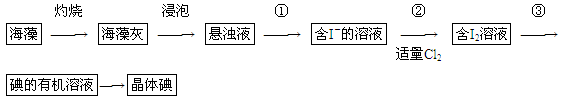 ��1��ʵ������۵�������________��������Ҫ��������Ϊ________��
��1��ʵ������۵�������________��������Ҫ��������Ϊ________��
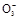 ��S
��S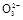 ��S
��S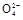 �����е����������,�ձ�ͬѧ���ʵ����������,��¼����:
�����е����������,�ձ�ͬѧ���ʵ����������,��¼����: