��Ŀ����
��1�������������У�4�۵������������������л�ԭ�ԣ������Լ�����ˮ��Na2S��Һ��
Na2SO3��Һ��ϡ���ᡢNaOH��Һ����ˮ��
��1��Ҫ֤��Na2SO3���л�ԭ�ԣ�Ӧѡ�õ��Լ���________�������������� ________����Ӧ�ķ���ʽΪ___________________________
��2��ʵ������ȡ�����Ļ�ѧ����ʽ�� ������Ϊ���ʣ����ϳ����Լ���Cl2�У�HCl�� ��
��3����SiO2��ȡ�ֹ�Ļ�ѧ����ʽ
��4����ag��CO��H2��ɵĻ��������������O2�г��ȼ�պ����ɵ����в���ͨ��������Na2O2���壬Na2O2�������ӵ�����Ϊ ag ���� ���� ������
Na2SO3��Һ��ϡ���ᡢNaOH��Һ����ˮ��
��1��Ҫ֤��Na2SO3���л�ԭ�ԣ�Ӧѡ�õ��Լ���________�������������� ________����Ӧ�ķ���ʽΪ___________________________
��2��ʵ������ȡ�����Ļ�ѧ����ʽ�� ������Ϊ���ʣ����ϳ����Լ���Cl2�У�HCl�� ��
��3����SiO2��ȡ�ֹ�Ļ�ѧ����ʽ
��4����ag��CO��H2��ɵĻ��������������O2�г��ȼ�պ����ɵ����в���ͨ��������Na2O2���壬Na2O2�������ӵ�����Ϊ ag ���� ���� ������
��1��Na2SO3 ��ˮ ��ˮ��ɫ Na2SO3+ Br2 + H2O = Na2SO4+2HBr
Na2SO3 + Br2 + H2O =H2SO4 + 2NaBr ��2�� MnO2 +4HCl(Ũ) MnCI2 + Cl2��+2H2O ����ʳ��ˮ��3��SiO2 + 2C
MnCI2 + Cl2��+2H2O ����ʳ��ˮ��3��SiO2 + 2C Si + 2CO ��4�� =
Si + 2CO ��4�� =
Na2SO3 + Br2 + H2O =H2SO4 + 2NaBr ��2�� MnO2 +4HCl(Ũ)
 MnCI2 + Cl2��+2H2O ����ʳ��ˮ��3��SiO2 + 2C
MnCI2 + Cl2��+2H2O ����ʳ��ˮ��3��SiO2 + 2C Si + 2CO ��4�� =
Si + 2CO ��4�� =�����������1��Ҫ֤��Na2SO3���л�ԭ�ԣ�Ӧѡ�õ��Լ���Na2SO3����ǿ�������Ե���������ˮ�������ķ�Ӧ�ǣ�Na2SO3+ Br2 + H2O = Na2SO4+2HBr������Ϊ��ˮ��ɫ����2�� ʵ������ȡ�����Ļ�ѧ����ʽ��MnO2 +4HCl(Ũ)
 MnCI2 + Cl2��+2H2O���������õ�Ũ���ᷴӦ��ȡ�ġ�Ũ�����лӷ��ԣ���������ȡ��Cl2�к�������HCl���塣��ȥ�ķ����ǰѻ������ͨ�뵽����NaClˮ�У�HCl�������ܽ���ˮ�ж��õ���ȥ��������ӦCl2+H2O
MnCI2 + Cl2��+2H2O���������õ�Ũ���ᷴӦ��ȡ�ġ�Ũ�����лӷ��ԣ���������ȡ��Cl2�к�������HCl���塣��ȥ�ķ����ǰѻ������ͨ�뵽����NaClˮ�У�HCl�������ܽ���ˮ�ж��õ���ȥ��������ӦCl2+H2O H++Cl-+HClO.��Ϊˮ�к���NaCl����HCl���������Cl-���������������Ũ�ȣ�ʹ��ѧƽ�������ƶ����ּ�����Cl2���ܽ⡢��Ӧ���ġ��ﵽ�˼ȳ�ȥ����Ҳ�����ٱ��ᴿ�����ʱ�������3����SiO2��ȡ�ֹ�Ļ�ѧ����ʽΪSiO2 + 2C
H++Cl-+HClO.��Ϊˮ�к���NaCl����HCl���������Cl-���������������Ũ�ȣ�ʹ��ѧƽ�������ƶ����ּ�����Cl2���ܽ⡢��Ӧ���ġ��ﵽ�˼ȳ�ȥ����Ҳ�����ٱ��ᴿ�����ʱ�������3����SiO2��ȡ�ֹ�Ļ�ѧ����ʽΪSiO2 + 2C Si + 2CO����4��2H2+O2
Si + 2CO����4��2H2+O2 2H2O��2Na2O2+2H2O=4NaOH+O2����2CO+O2
2H2O��2Na2O2+2H2O=4NaOH+O2����2CO+O2 2CO2��2Na2O2+2CO2=2Na2CO3+O2���ɼ���H2��COȼ�����ĵ������뷴Ӧ������Na2O2��Ӧ�ų�������������ȡ����������ӵ��������Ƿ�Ӧ��H2��CO�������͡���������������Ϊag����ȼ�ղ�����������Ʒ�Ӧ������������ӵ�Ҳ��ag.2SO3��Na2O2�Ļ�ѧ���ʼ�Si ��Cl2���Ʒ����ʵij�ȥ��֪ʶ��
2CO2��2Na2O2+2CO2=2Na2CO3+O2���ɼ���H2��COȼ�����ĵ������뷴Ӧ������Na2O2��Ӧ�ų�������������ȡ����������ӵ��������Ƿ�Ӧ��H2��CO�������͡���������������Ϊag����ȼ�ղ�����������Ʒ�Ӧ������������ӵ�Ҳ��ag.2SO3��Na2O2�Ļ�ѧ���ʼ�Si ��Cl2���Ʒ����ʵij�ȥ��֪ʶ��
��ϰ��ϵ�д�
 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�
�����Ŀ

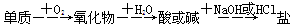 ����ʵ���������ʼ�ֱ��ת����Ԫ��
����ʵ���������ʼ�ֱ��ת����Ԫ��