��Ŀ����
(ÿС��2�֣���3С��ȫ�ԲŸ��֣���10��)W��X��Y��Z��ԭ���������������ͬһ��ͬ��Ԫ�أ�W��X�ǽ���Ԫ�أ�Y��Z�Ƿǽ���Ԫ�ء�
(1��W��X���Ե�����������Ӧ��ˮ����������Ӧ���κ�ˮ���÷�Ӧ�����ӷ���ʽΪ____________________��
(2��W��Y ���γɻ�����W2Y���û�����ĵ���ʽΪ______________��
(3��X��������ˮ��Һ��______�ԣ������ӷ���ʽ����ԭ��________________________��
(4��Y�ĵͼ�������ͨ��Z���ʵ�ˮ��Һ�У�������Ӧ�Ļ�ѧ����ʽΪ_________________��
(5��Z�����������Ϊ��ɫҺ�壬0.25 mol��������һ����ˮ��ϵõ�һ��ϡ��Һ�����ų�QkJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
(1��Al(OH)3+OH-��AlO2-+2H2O��
(2��![]()
(3���� Al3++3H2O![]() Al(OH)3+3H+��
Al(OH)3+3H+��
(4��SO2+Cl2+2H2O��H2SO4+ 2HCl��
(5��Cl2O7��l��+H2O��l����2HClO4(aq)����H��-4QkJ?mol-1��

 �߽�������ϵ�д�
�߽�������ϵ�д���ÿ��1�֣���4�֣���ͨ��״���£���ͬѧȡ1 mol H2O���ȵ�100��ʱ��Һ̬ˮ������Ϊˮ��������ͼ��ʾ�����ù������� �仯��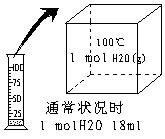
�ڱ���ѹǿ���������£�ˮ��������� 
���������������������22.4L��
����ͬѧ��H2��O2��ȼ�յ�ʵ�飬��ʵ��������� �仯���ڸñ仯�����У�һ��������ȵ��� ������ţ���
| A����Ӧ�������Ŀ�������������Ŀ | B����Ӧ��ԭ�������ʵ�����������ԭ�������ʵ��� |
| C����Ӧ���������������������� | D����Ӧ���������������� |
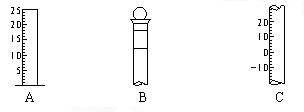
����д���������������ƣ� A ��B ��C ��
������B�ϱ���� ������ţ���
������ ���¶�
 �ۿ̶��� ��Ũ�� ���ݻ�
�ۿ̶��� ��Ũ�� ���ݻ��Ǽ�������B�Ƿ�©ˮ�ķ�����
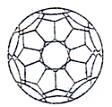 ��(3)С���е�CxҲ������һ�ָ���ϩ�����(3)С��Cx�ṹ������κ������εĸ����ֱ���____________�� ������ʾ��ŷ��������������+����������=2��
��(3)С���е�CxҲ������һ�ָ���ϩ�����(3)С��Cx�ṹ������κ������εĸ����ֱ���____________�� ������ʾ��ŷ��������������+����������=2��