��Ŀ����
��10�֣�ijУ��ѧʵ��С������������������480 mL 0.5 mol/L��NaOH��Һ�����ڵ���ˮ�ʼ�⡣�Իش�������⣺
(1)��С��ͬѧѡ��________ mL������ƿ��
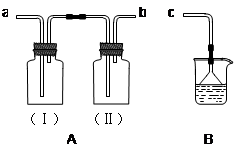
(2)�����������ͼ����ʾ������ͼ����ʾ����Ӧ��ͼ���е�________(��ѡ����ĸ)֮�䡣

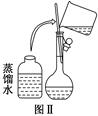
(3)��С��ͬѧӦ��ȡNaOH����________g
(4)���в�������������Һ��Ũ�ȴ�С�к�Ӱ�죿
��ת������Һ��δϴ�Ӳ��������ձ���Ũ��________(�ƫ����ƫС������Ӱ�족����ͬ)��
������ƿ��ԭ������������ˮ��Ũ��________��
(1)��С��ͬѧѡ��________ mL������ƿ��
(2)�����������ͼ����ʾ������ͼ����ʾ����Ӧ��ͼ���е�________(��ѡ����ĸ)֮�䡣


| A���ں͢� | B���ٺ͢� | C���ܺ͢� | D���ۺ͢� |
(4)���в�������������Һ��Ũ�ȴ�С�к�Ӱ�죿
��ת������Һ��δϴ�Ӳ��������ձ���Ũ��________(�ƫ����ƫС������Ӱ�족����ͬ)��
������ƿ��ԭ������������ˮ��Ũ��________��
��500��2�֣� ��C��2�֣� ��10.0��2�֣� ��ƫС��2�֣���Ӱ�죨2�֣�
��1����������ƿ�Ĺ��û��480ml�ģ�����Ӧ��ѡ��500ml����ƿ��
��2���������ƹ��̿�֪��������ƿ����Һͨ����Ͼ��Ⱥ���Ҫ������ƿ�м�������ˮ��Ӧ�ô�ѡC��
��3��500ml 0.5 mol/L��NaOH��Һ���������Ƶ����ʵ�����0.25mol��������0.25mol��40g/mol��10.0g��
��4������c��n/V��֪�����δϴ�Ӳ��������ձ�������Һ�����ʵ����ʵ������٣�Ũ��ƫС������ƿ�к�������ˮ��Ũ�Ȳ�Ӱ�졣
��2���������ƹ��̿�֪��������ƿ����Һͨ����Ͼ��Ⱥ���Ҫ������ƿ�м�������ˮ��Ӧ�ô�ѡC��
��3��500ml 0.5 mol/L��NaOH��Һ���������Ƶ����ʵ�����0.25mol��������0.25mol��40g/mol��10.0g��
��4������c��n/V��֪�����δϴ�Ӳ��������ձ�������Һ�����ʵ����ʵ������٣�Ũ��ƫС������ƿ�к�������ˮ��Ũ�Ȳ�Ӱ�졣

��ϰ��ϵ�д�
�����Ŀ