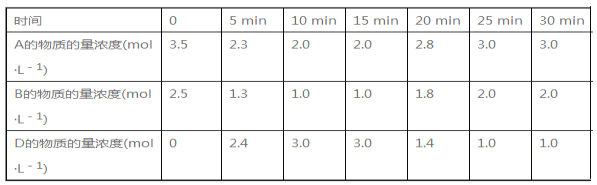
��Ŀ����
����Ŀ������ͼ��ʾװ�ý���ʵ�飨�г�����ʡ�ԣ�����Һ��A��μ��뵽����B�У��ش��������⣺
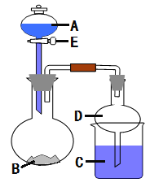
��1��ͼ��ʢ��Һ��A����������Ϊ_________________��Dװ����ʵ���е�������_______________________��
��2����AΪŨ���ᣬBΪKMnO4��C��ʢ��KI-������Һ������E��C�е�����Ϊ____________��C�з�����Ӧ�����ӷ���ʽΪ__________������ͨ������C�У��㹻����ʱ�����C����Һ����ɫ��ʧ��������Ϊ����Һ��I2�ܱ�Cl2����ΪHIO3��д���÷�Ӧ�Ļ�ѧ����ʽ�� ______��
��3����AΪŨ��ˮ��BΪ��ʯ�ң�C��ʢ��AlCl3��Һ������E���㹻����ʱ���C�е�������__________��C�з�����Ӧ�����ӷ���ʽΪ_________��
���𰸡���Һ©�� ������ ��Һ����ɫ��Ϊ��ɫ Cl2 + 2I = I2 + 2Cl- I2 + 5Cl2 + 6H2O = 2HIO3 + 10HCl �а�ɫ��״�������� Al3+ + 3NH3H2O = Al(OH)3��+3NH4+
��������
��1�����������ṹ����;����װ���в�����������ˮ�Ӵ����ܻ��γ���ѹ�
��2����AΪŨ���ᣬBΪKMnO4����A��B���Ʊ���������ͨ��C����KI����������ԭ��Ӧ���ݴ˷�������
��3����AΪŨ��ˮ��BΪ��ʯ�ң�������ɰ���������ˮ�γ�һˮ�ϰ���һˮ�ϰ�Ϊ���������һˮ�ϰ����Ȼ������Ʊ���������������
��1��ͼ��ʢ��Һ��A����������Ϊ��Һ©����Dװ����ʵ���е������Ƿ�������
�ʴ�Ϊ����Һ©������������
��2��KMnO4��Ũ���ᷴӦ�����������������������⻯����Һ���ɵⵥ�ʣ������۱�����������Ϊ��Һ����ɫ��Ϊ��ɫ�����ӷ�Ӧ����ʽΪ��Cl2 + 2I = I2 + 2Cl-������ͨ��������I2�ᱻ��Cl2����ΪHIO3������������ԭ��Ӧ���ɿ�֪���仯ѧ����ʽΪ��I2 + 5Cl2 + 6H2O = 2HIO3 + 10HCl��
�ʴ�Ϊ����Һ����ɫ��Ϊ��ɫ��Cl2 + 2I = I2 + 2Cl-��I2 + 5Cl2 + 6H2O = 2HIO3 + 10HCl��
��3��Ũ��ˮ����ʯ�ҿɿ�����ȡ��������������ˮ�γɵ�һˮ�ϰ������Ȼ�����Ӧ����������������������Ϊ���а�ɫ��״�������ɣ���Ӧ�����ӷ���ʽΪ��Al3+ + 3NH3H2O = Al(OH)3��+3NH4+��
�ʴ�Ϊ���а�ɫ��״�������ɣ�Al3+ + 3NH3H2O = Al(OH)3��+3NH4+��

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�